- Submissions

Full Text
Novel Research in Sciences
Influence of Hyperbaric Conditions on Serum Cortisol Levels and Alpha- Amylase in Saliva
Yordanova Mariana1,3*, Shopov Nikola2 and Daniela Gerova3
1Military Medical Academy, Multiprofile Hospital for Active Treatment, Bulgaria
2Military Medical Academy, Department of Aviation and Marine Medicine, Bulgaria
3Paraskev Stoyanov Medical University Varna, Department of Clinical Laboratory, Bulgaria
*Corresponding author: Yordanova Mariana, Military Medical Academy, Multiprofile Hospital for Active Treatment, Head Clinical laboratiory and immunology, Bulgaria
Submission: July 13, 2022;Published: July 29, 2022
.jpg)
Volume11 Issue4July , 2022
Abstract
Introduction: A high level of physiological stress characterizes diving. Serum cortisol and Salivary Alpha-
Amylase (sAA) values are markers closely related to dynamics and adaptation under different stress
conditions.
The aim is to determine serum cortisol and salivary alpha-amylase levels as stress markers affected by
diving and hyperbaric conditions.
Material and methods: Twenty-two military divers with an average age of 34.4±4.1 years were studied,
with more than five years of diving experience, conducting 42-meter dives into the hyperbaric chamber
with a bottom time of 35 minutes. Serum levels of cortisol and sAA were determined twice (before and
15min after decompression).
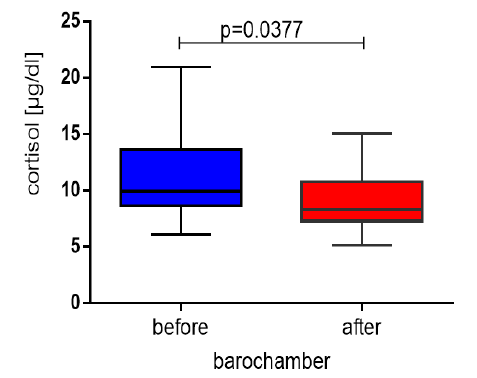
Results and discussion: Contrary to expectations, the mean values of cortisol before compression in the
chamber were higher than after surfacing, and the difference was statistically significant (11.82±4.42μg/
dl, respectively 9.09±2.73μg/dl; p=0.0377). After decompression, the salivary values of sAA increased
compared to the pre-chamber, but they were not statistically significant (77.01±54.28, respectively,
54.69±36.27 U/ml; p=0.1817).
We hypothesize that subjects have adapted in the stress response during dive into the chamber and that
cortisol levels paradoxically do not increase after the descent. Increased sAA levels are most likely due
to the effects of hyperbaric oxygenation and dehydration, decreased salivary secretion, and to a lesser
extent, stress.
Conclusion: The conditions in the chamber are not adequate to the extreme stress conditions of the
actual underwater descent, where the stress factor is hypothermia, fatigue, anxiety, poor visibility and
more. A real-sea study will provide more accurate information on the occurrence of diving stress.
Keywords:Divers; Stress; Cortisol; Alpha-amylase; Saliva
Introduction
The human capacity to tolerate environmentally induced stress depends on a complex network of biological processes and mediators that play a role in maintaining homeostasis. Acute stress mediators are released and become active within seconds of the initial impact of the stressor and provide rapid adaptive responses and coping strategies. Several biomarkers reflecting the activation of the Hypothalamus-Pituitary-Adrenal (HPA) axis and the Sympathetic-Adrenal-Medullary (SAM) axis can be quantified to assess stress levels [1]. A high level of physiological stress characterizes diving activity. During diving descent, several factors such as being in cold water, hyperoxia, intravascular nitrogen bubble formation, physical exertion, psychological stress, etc., lead to a wide range of health risks and the involvement of physiological adaptive mechanisms [2,3]. Increased hydrostatic pressure and depth of descent are stressors and affect the body by altering the levels of various hormones and enzymes [4].
Salivary secretion and modification are regulated by the autonomic nervous system, undergo circadian variations and depend on various stimuli such as smell, taste, pain, feeding, emotional state, and receptor influences at the level of the autonomic nervous system [5,6]. Activating the Parasympathetic Nervous System (PNS) stimulates blood flow to the glands and produces large amounts of watery saliva (rich in ions and enzymes). Conversely, stimulation of the Sympathetic Nervous System (SNS) produces small amounts of saliva containing more proteins [7]. Saliva is a biological material easily obtained through an accessible, non-intrusive, painless procedure. It contains a variety of low molecular weight substances, enzymes, hormones, antibodies, antimicrobial compounds and growth factors [8-10]. One of the essential salivary proteins of glandular origin is sAA, which accounts for about 40 to 50% of total salivary protein. A beta-adrenergic stimulus initiates salivary amylase production. The activity of sAA varies widely, between 30,000 and 800,000 U/L, due to reverse resorption of water in the striated duct and osmotic transport from the parotid duct, and is considered a reliable marker of serous cell function [11].
A growing number of observations show the relationship between salivary amylase secretion and physical and mental state. Increases in enzyme activity have been found under conditions of psycho-emotional stress, during strenuous exercise and sports, running, cycling, exposure to cold [12] and negative emotions. This makes it an apt marker to monitor changes in the body upon activation of the SNS under stress conditions. The so-called stress hormones are particularly interesting, with cortisol as the primary representative. According to Hans Selye’s concept, it is a glucocorticoid synthesized by the adrenal cortex and is a primary adaptive hormone to changing environmental conditions. Cortisol has diurnal variations, with the highest levels occurring around 06:00-08:00 and the lowest levels around midnight. When the HPA axis is activated, cortisol secretion from the adrenal cortex increases. Chronic stress conditions lead to oxidative stress, lowers immunity, increase blood pressure, disrupts carbohydrate balance, increase abdominal fat deposition, and decrease muscle mass.
Recently, several authors have investigated serum or salivary biomarkers to assess and objectify stress responses in different groups (athletes, divers, etc.) and their adaptability to changing environmental conditions.
Aim
Our study aimed to determine how serum cortisol and salivary alpha-amylase levels are affected in hyperbaric conditions as markers reflecting stress in а diving activity.
Material and Method
The persons who form our group are undergoing a routine health expert examination at the Military Medical Academy - Varna. The study was conducted from September 2017-June 2019 and was approved by the Research Ethics Committee of MU-Varna with protocol No. 64/13.07.2017. The twenty volunteer military divers studied were men with an average of 10 years of diving experience (5 to 16) and a corresponding standard practice of 2000 (500- 3500) hours. The divers gave informed consent and signed in their own hands according to the European Code of Ethics requirements for research integrity and the Declaration of Helsinki. The persons studied underwent routine tests regarding the specifics of their occupational activity. A simulated depth descent into a hyperbaric chamber was performed to a depth of 42 meters, with a bottom stay of 35 minutes and a total decompression time of 67 minutes. Biological material was collected twice (before the barochamber and 15 minutes after decompression) to determine serum cortisol and sAA levels.
Sampling was consistent with standard preanalytical requirements and circadian rhythms of serum cortisol and salivary amylase secretion. A simulated depth descent into a hyperbaric chamber was performed to a depth of 42 meters, with a bottom stay of 35 minutes and a total decompression time of 67 minutes. Biological material was collected twice (before the hyperbaric chamber and 15 minutes after decompression) to determine serum cortisol and sAA levels.
Sampling was consistent with a standard preanalytical requirement and circadian rhythms of serum cortisol and salivary amylase secretion. Blood samples were collected in a suitable certified vacutainer (anticoagulant-free gel separator) to study biochemical parameters. Centrifugation was performed for 10 minutes at 3500 rpm, and cortisol was examined in the separated blood serum using the chemiluminescent method on an Access2 immunochemical analyzer (Beckman Coulter-USA). Saliva was collected in special sterile graduated polystyrene containers with a conical bottom. Subjects were instructed not to eat, drink coffee or other tonic beverages, or chew gum in the 30 minutes preceding the study. Five minutes before the examination, the mouth was rinsed twice with saline or table/mineral water by gargling for 10 seconds. Spontaneously collected saliva in the lower part of the oral cavity (without suctioning), by passive repeated excretion, is collected in the container to a volume (2÷3ml) within 5-6 minutes. The biological samples are centrifuged at room temperature at 2500rpm for 5 minutes. In the supernatant determined sAA activity using an Indiko Plus biochemical analyzer (USA) with a Thermo Scientific reagent. This kinetic-colourimetric method was chosen because it has a wide linear range and the ability for high-level automatic dilution (10-24000 U/L).
Statistical analysis of the obtained results was performed using GraphPad Prism v.6.0 for Windows software, GraphPad Software, La Jolla California USA using standard statistical methods: descriptive, analysis of variance-to assess the central tendency and variance characteristics of the data, Kolmogorov-Smirnov test to determine the type of distribution of the data in the studied groups, parametric t-test analysis and non-parametric Mann-Whitney test. All statistical analyses performed assumed an acceptable significance level, rejecting the null hypothesis of p<0.05.
Results
The physiological characteristics of the subjects included in the study are given in Table 1. Salivary amylase testing is carried out in unstimulated saliva twice - before entering the pressure chamber and 15 minutes after decompression. The values are given in Figure 1. Blood samples for serum cortisol testing are taken in the same setup twice, similar to saliva collection. The values are presented in Figure 2.
Table 1:Biometric data of divers (n = 20).

Figure 1: Values of sAA in the studied divers before the barochamber and after decompression.
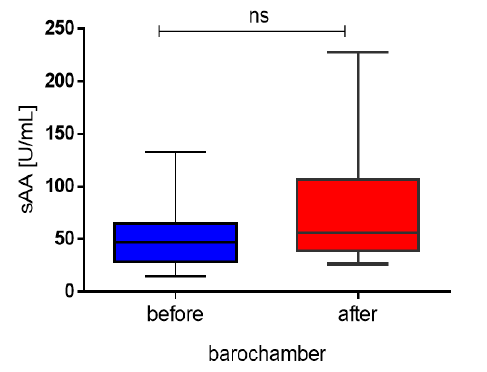
Figure 2: Cortisol values in the studied divers before the barochamber and after decompression. The comparative analysis was carried out using the t-test.
Discussion
Psychological stress and anxiety, cold as an environmental factor, and physical exertion are well-known stimulants altering cortisol values. Activation of the HPA and SAM axes are the two major components involved in the stress response leading to measurable changes in blood or salivary stress hormone levels. Diving activity is characterized by a high level of physiological stress [2-4], studying divers who performed SCUBA dives in open water with a temperature of 24-27 ⁰C to a depth of 30 meters, found significant differences in cortisol levels from baseline values (p<0.001). They found that this increase was independent of the depth of descent and the anthropometric and physical characteristics of the divers. Coetzee N [13], in an investigation of salivary cortisol levels in open water trainee divers, found a significant increase in levels after diver descent compared to control baseline values. Apparently, the adaptive physiological response to stress resulting from the anticipation of the novel experience and the inexperience of the subjects is the cause of these data. Moderate increases in cortisol levels positively affect the body as they increase energy levels, enhance memory functions, and maintain homeostasis after a stressful event. At the same time, high hormone values have an adverse effect with a higher risk of developing panic reactions, poor orientation and coping with the environment.
While in another study, only performed in a hyperbaric chamber with eight professional divers placed in hyperbaric conditions (253kPa) for 60 minutes, a significant decrease (p=0.001) in cortisol was found [14]. Similar findings were made by Coetzee N [13] and Tikkinen J [15], finding that plasma or salivary cortisol levels decreased during exposure to elevated pressure in a hyperbaric chamber. Still, they increased after breath-hold diving in a natural open water setting. These opposing findings regarding the effects on cortisol find their physiological explanation in the type and method of diving and the involvement of different adaptive mechanisms in the body. Hypoxemic stress appears to be the most impactful stressor in breath-hold diving. It leads to an increase in stress hormone values, compared to the lowering effect of hyperoxia in hyperbaric chamber dives. Other factors such as cold, increased hydrostatic pressure, and hyperbaric gas inspiration appear to be more secondary stressors when diving in a real-life setting [16]. The studies mentioned above correspond with the statistically significant reduction in serum cortisol levels found in our research (9.09±2.73μg/dl versus baseline values of 11.82±4.42μg/dl; p=0.0377) measured during a simulated deep descent into a hyperbaric chamber at 42m for 35 minutes. Hyperoxia stimulated the sympathetic nervous system and promoted vasoconstriction, activating mechanoreceptors in the aortic arch and carotid sinuses. Their signals, mediated by the CNS response, lead to the suppression of sympathetic activity and the promotion of parasympathetic activity [17]. Probably the effect of increased partial pressure of oxygen (pO2) in the hyperbaric chamber acts as a stress marker reducing factor.
The autonomic nervous system regulates salivary gland secretion. The acini are responsible for saliva’s volume and flow rate, while the glandular duct cells, through reverse resorption or secretion processes, determine its composition [18]. Activation of the sympathetic nervous system causes the production of proteinrich saliva at low flow rates. In contrast, the parasympathetic nervous system produces large amounts of watery saliva (rich in ions and enzymes). Alpha-amylase is secreted mainly by the most prominent salivary glands (parotid gland) and possesses not only digestive but also antibacterial action in the oral cavity. The relationship between salivary amylase secretion and physical and mental state has been demonstrated. An increase in enzyme activity is observed under conditions of psycho-emotional stress, during strenuous exercise and sports, running, cycling, exposure to cold, hyperbaric conditions and negative emotions [12,19-21].
A characteristic feature of sAA is the wide variation of the enzyme activity in the normal range and its dependence on the diet regimen. Saliva is still a non-routinely studied biological material, and there are no uniform reference limits for a number of biochemical parameters, including alpha-amylase. The reference range (17.62÷79.95 U/ml) that we used to estimate the values obtained in the subjects of unstimulated saliva was determined in our laboratory according to the recommendations of the Expert Group on Reference Value Theory of the International Federation of Clinical Chemistry (IFCC) and reflect specific regional and traditional conditions of lifestyle and culture of individuals from the Bulgarian population [22]. In the present study, there was an increase in salivary amylase activity 15 minutes after decompression compared to baseline values, although the differences were not statistically significant (77.01±54.28U/ml and 54.69±36.27U/ml, respectively; p=0.1817). The enzyme values do not exceed reference limits because divers who participated in the study were experienced professionals with over five years of diving experience. It is unlikely that mental stress was the main factor in the increase in sAA. In addition, they were at rest during the hyperbaric exposure in the hyperbaric chamber, which also rules out the physical activity as a factor in the enzyme increase. In a study by Deb et al. (2021), underwater descent where greater physical exertion was required, also similar to our results, observed no significant changes in IL- 6, cortisol and alpha-amylase. The increased sAA activity is most likely due to the effects of hyperbaric oxygenation and dehydration and a reduction in salivary secretion rather than being due to stress.
Conclusion
The conditions in the hyperbaric chamber are not adequate for the highly stressful conditions of the actual underwater descent, where the stressors are hypothermia, fatigue, anxiety, poor visibility, etc. Research in a natural environment will give more reliable information about the occurrence of stress during diving descents. Salivary alpha-amylase levels best measure the activation of the sympathoadrenal system in response to acute stress. While regarding long-term or chronic stress, cortisol (in saliva or serum) is most relevant, which is secreted as a stimulus when the hypothalamus-pituitary-adrenal axis is activated.
Limitations
Our study was conducted with a small sample size and substantiates the use of salivary alpha-amylase and serum cortisol levels as markers of psychogenic stress and risk in diving activity. Cortisol testing could also be done in saliva, which would facilitate non-invasive sampling. Additional studies involving more participants and a survey conducted in a natural underwater environment may shed more light on the issue.
References
- Monnoyer R, Lautridou J, Deb S, Hjelde A, Eftedal I, et al. (2021) Using salivary biomarkers for stress assessment in offshore saturation diving: A pilot study. Front Physiol 12: 791525.
- Obad A, Palada I, Valic Z, Ivancev V, Bakovic D, et al. (2007) The effects of acute oral antioxidants on diving‐induced alterations in human cardiovascular function. J Physiol 578(Pt 3): 859-870.
- Cialoni D, Pieri M, Balestra C, Marroni A (2017) Dive risk factors, gas bubble formation, and decompression illness in recreational scuba diving: Analysis of DAN Europe DSL data base. Front Psychol 8: 1587.
- Zarezadeh R, Azarbayjani MA (2014) The effect of air scuba dives up to a depth of 30 metres on serum cortisol in male divers. Diving Hyperb Med 44(3): 158-160.
- Nater UM, La Marca R, Florin L, Moses A, Langhans W, et al. (2006) Stress-induced changes in human salivary alpha-amylase activity-associations with adrenergic activity. Psychoneuroendocrinology 31(1): 49-58.
- Alhajj M, Babos M (2011) Physiology, salivation. Stat Pearls Publishing LLC, Tampa, Florida, United States.
- Chiappin S, Antonelli G, Gatti R, De Palo EF (2007) Saliva specimen: A new laboratory tool for diagnostic and basic investigation. Clin Chim Acta 383(1-2): 30-40.
- Humphrey SP, Williamson RT (2001) A review of saliva: Normal composition, flow, and function. J Prosthet Dent 85(2): 162-169.
- Malamud D (2011) Saliva as a diagnostic fluid. Dent Clin North Am 55(1): 159-178.
- Nunes LA, Mussavira S, Bindhu OS (2015) Clinical and diagnostic utility of saliva as a non-invasive diagnostic fluid: A systematic review. Biochem Med (Zagreb) 25(2): 177-192.
- Almståhl A, Wikström M, Groenink J (2001) Lactoferrin, amylase and mucin MUC5B and their relation to the oral microflora in hyposalivation of different origins. Oral Microbiol Immunol 16(6): 345-352.
- Hensten A, Jacobsen N (2019) Salivary alpha amylase as a stress biomarker. OSP J Dent Sci 1(1): 1-6.
- Coetzee N (2011) Measurement of heart rate variability and salivarycortisol levels in beginner scuba divers. African Journal for Physical, Health Education, Recreation and Dance 17(4): 729-741.
- Lund V, Kentala E, Scheinin H, Klossner J, Koskinen P, et al. (1999) Effect of hyperbaric conditions on plasma stress hormone levels and endothelin-1. Undersea Hyperb Med 26(2): 87-92.
- Tikkinen J, Hirvonen A, Parkkola K, Siimes MA (2011) The effects of increased pressure, variation in inspired gases and the useof a mask during dry chamber dives on salivary cortisol inprofessional divers . Diving Hyperb Med 41(4): 211-215.
- Marlinge M, Coulange M, Fitzpatrick RC, Delacroix R, Gabarre A, et al. (2019) Physiological stress markers during breath-hold diving and scuba diving. Physiol Rep 7(6): e14033.
- Goyal A, Chonis T, Bhyan P, Cooper JS (2021) Hyperbaric cardiovascular effects. Stat Pearls Publishing LLC, Tampa, Florida, United States.
- de Paula F, Teshima THN, Hsieh R, Souza MM, Nico MMS, et al. (2017) Overview of human salivary glands: Highlights of morphology and developing processes. Anat Rec (Hoboken) 300(7): 1180-1188.
- Nater UM, Skoluda N, Strahler J (2013) Biomarkers of stress in behavioural medicine. Curr Opin Psychiatry 26(5): 440-445.
- Nater UM, La Marca R, Erni K, Ehlert U (2015) Alpha-amylase activity in blood increases after pharmacological, but not psychological, activation of the adrenergic system. PLoS One 10(6): e0130449.
- Ali N, Nater UM (2020) Salivary alpha-amylase as a biomarker of stress in behavioral medicine. Int J Behav Med 27: 337-342.
- Yordanova M (2021) Comparative study of biochemical markers in different biological matrixes in patients with chronic diseases of the gastro-intestinal tract, PhD Thesis, Medical University Varna, Bulgaria.
© 2022 Yordanova Mariana. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






