- Submissions

Full Text
Novel Research in Sciences
Analytical Aspects of Essential Compounds and Nutritional Impact of Economic Important Fish Species (Heterotis niloticus and Macolor niger) in Water Bodies
Ayofe M Hammed1*, Albert O Amosu2, Folalu A Awe1, Lucas O Dalmeida2 and Bukky R Mendes1
1Department of Fisheries, Faculty of Science, Lagos State University, P.O. Box 0001, LASU Post office, Ojo, Lagos, Nigeria
2Department of Agricultural Science, Lagos State University of Education, Oto/Ijanikin, PM.B 007 Festac Town, Lagos, Nigeria
*Corresponding author: Ayofe M Hammed, Department of Fisheries, Faculty of Science, Lagos State University, P.O. Box 0001, LASU Post office, Ojo, Lagos, Nigeria
Submission: June 06, 2022;Published: July 07, 2022
.jpg)
Volume11 Issue3June, 2022
Abstract
The biological composition of fish food and water can affect the minerals and nutrient composition of the fish, showing the relationship between the fish, its habitat and what they eat. It has necessitated the research on the amino acid, mineral and proximate compositions of Heterotis niloticus and Macolor niger from coastal water bodies to investigate the nutritional status of these two prominent and economic important fish species. A total of 120 specimens of weight between 500 and 700g were examined. Water and benthic sediments from the three locations were analyzed for quality assessment (Badagry creek, Ikorodu and Lagos island area of Lagos, Nigeria). The amino acid and Proximate compositions were determined by method describes by Association of Analytical Chemist (AOAC). Mean body mineral constituent in the fish differed significantly (p<0.05) between the two species except from arsenic, lead and nickel. Lagos lagoon has the lowest percentage of iron in the water (0.08±0.00), while Ikorodu coastal water has the largest percentage (0.31±0.00), fat constituents values of 16.86±1.08 (H. niloticus) and 0.99±0.08 (M. niger) were detected in both species. Among the 8 essential amino acids detected; Leucine has the lowest mean value of 7.93±.04 in H. niloticus while M. niger had the highest concentration of 9.25±0.28 in H. niloticus and M. niger. All the amino acids were discovered to be significantly different at p<0.05. both fishes contains all the essential amino acids in the permissible and appreciable concentrations. Due to the abundant nutrients found in the two species examined, we hereby recommend H. niloticus and M. niger in the studied coastal waters for human consumption.
Keywords: Keywords: Proximate; Minerals; Amino acids; Heterotis niloticus; Macolor niger
Introduction
Fish are potential source of animal protein and significant nutrients in human diets. Fish meat are made to contain slow lipids and higher water than bee for poultry and is well favored than white or red meats [1]. The nutritional value of fish tissues constitutes moisture, dry matter, protein, lipids, vitamins, minerals in addition to the caloric value of the fish. Minerals are important and required nutrients and are components of metabolic enzymes that contribute to the biomass of the fish [2]. From nutritional point of view, fish composite of very high nutritional quality which is rich in most vitamins, proteins, minerals, fats and amino acids and is a nutritious part of human diet. An idea which had been justified by some biological experiments that is nutritionally equivalent to meat, milk and eggs FAO and [3].
Despite the recognition of the essential roles of minerals for various life processes, research on mineral and trace element nutrition of fish has progressed rather slowly. Although about 29 of the 90 naturally occurring inorganic elements are considered to be essential for all farmed animals including fish, only few of them have been studied in detail in fish [4]. Dietary requirements are established form macro-minerals such as Ca, K, Mg, Na, P and micro-minerals such as Cu, Fe, I, Mn, Se, Zn for one or more fish species [5]. Studies have dealt with the functions, deficiency, availability, utilization, toxicity, interaction with other nutrients and environment on the fore said minerals to several fish species [6-9]. It is recognized that nutrient requirement of an animal should be determined in terms of a specific response criterion at a given age, sex, weight gain and body composition [8]. This applies to studies on mineral and trace element requirements of fish as well. However, it is much more complicated in fish due to the close interaction with the aquatic environment unlike in terrestrial animals [10]. The factors that may affect the minimal dietary levels of mineral and trace element to fish can be one or a combination of the following: biological factors such as species, life stage, sex, trophic level, feeding habits and the nutritional status of the fish; dietary factors such as diet composition, availability and nutrient interactions; and environmental factors such as water mineral concentration, salinity and temperature of the rearing system [8,11,12]. The health status also affects the micronutrient and macronutrient content of fish tissues [13,14].
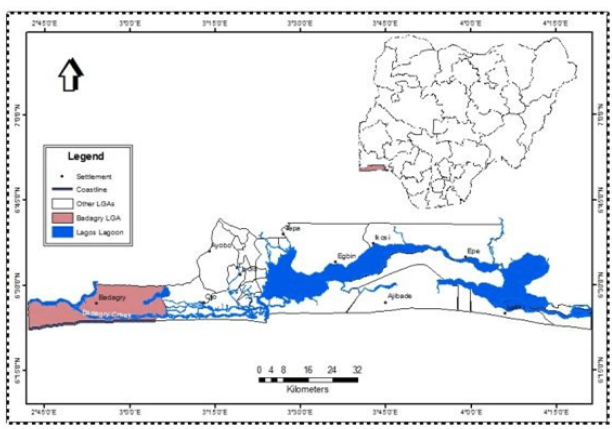
Dietary Amino Acids (AA) are crucial for fish as energy substrates, for endogenous protein synthesis and to regulate metabolic pathways. More than half of the AA consumed by fish may be deposited into body protein, and the requirement of Essential Amino Acids (EAA) corresponds to the AA tissue content [15]. Protein is a significant component of fish diets and is generally higher in carnivorous than herbivore fish. In salmonid diets, protein makes up 35-55% of the diet, with highest inclusion levels at early life stages [5]. Most of the AA are protein bound but can also be supplied in the form of crystalline AA to fulfill the AA requirement, as regulated by national legislation of feed additives, especially when using alternative protein sources [16]. Amino acid have traditionally been classified as essential (EAA) or nonessential (NEAA) relating to whether the organism can produce the Amino acid endogenously from the dietary NEAA. Recently, the term Functional Amino acid (FAA) have received more attention relating to AA that modulate key metabolic pathways thus affecting immune response, health, reproduction, cell signaling, animal welfare and more [17]. Amino acid classified as NEAA such as glutamine, glutamate and proline have been demonstrated to have functional properties in both fish and mammalian metabolism, suggesting that fish have requirement also for NEAA to obtain maximum performance [18,19]. In the case for NEAA, both the dietary content of the AA and its substrates are of importance. The AA here includes arginine, glutamate, glutamine, tryptophan, histidine, sulfur amino acids (SAA; methionine, cysteine and taurine) and branched chained AA (BCAA; leucine, isoleucine and valine). Notably, other AA including glycine, lysine, threonine and aromatic AA are also involved in metabolic pathways. The major aim of this study was to investigation the proximate, minerals and amino acids compositions of the Heterotis niloticus and Macolor niger. This study tend to compare the proximate, minerals and amino acids compositions of the most consumed fish species in some specific locations in Lagos (Figure 1).
Figure 1: Map showing Badagry creek, Lagos lagoon and Ikorodu coastal areas of Lagos State.
Material and Method
Study area
Studies were done emphasizing on three major areas which are The Badagry creek is approximately 60km long and 3km wide and lies between longitude 20 421 and 30 231E and latitude 60231 and 60281 N. It is part of a continuous system of lagoons and creeks along the coast of Nigeria from the border with the Republic of Benin to the Niger delta. Its water depth ranges from 1- 3m. The creek experiences two broad seasons: the dry season (December - May) and the wet season (June - November). Most of the year, it is characterized by fresh and slightly brackish water. The creek is approximately equidistant from the entrances of Lagos and Cotonou harbour. As a result, it is influenced by tides and floods from the Lagos Lagoon and Cotonou harbour through Lake Nokue and Lake Porto-Novo. Badore is situated in Ibeju Lekki, Lagos, Nigeria, its geographical coordinates are 6° 26’ 0” North, 3° 51’ 0” East and its original name (with diacritics) is Badore. Ikorodu is bounded to the south by the Lagos Lagoon, to the north by a boundary with Ogun State, and to the east by a boundary with Agbowa-Ikosi, a town in Epe Division of Lagos State. The town has grown significantly in the past 40 years and is divided into sixteen or seventeen “Ituns” or minor areas. The main industries in the town are trading, farming and manufacturing. Nearby major towns include Imota, Isiu, Liadi, Egbin, Ijede, Igbogbo and Bayeku, all of which constitute their own Local Council Development Area with their own traditional rulers (Obas).
Sample collection and preparation
20 fish each (120 in all) and between 500 and 700g was collected for analysis in each location. The samples were brought to the Department of Fisheries, Lagos State University Laboratory for digestion and water parameter testing. The fin, liver and flesh of the fish were removed and washed with ultra-pure water. 1g of each sample was taken, weighed and digested with Aqua rega in a flume tank on low heat. The digested samples were then stored in a sampling bottle and carried out for analysis at dISI analytical in Surulere, Lagos.
Reagents and standard solutions
Analytical reagent grade chemicals were used in all tests. Except otherwise stated, all reagents used for QC procedures were from Hach Chemical Company, USA. Reagents and certified reference standards were procured from authorised dealers: Analytical grade nitric acid, hydrochloric acid, sulphuric acid, Ethanol (AR;99.9%) and acetone (AR), were procured from Sigma- AldrichCo., USA. All the volumetric glass ware used was Type “A”. Calibrated micropipettes with range100-1000μl, and 1-5ml were used (where applicable). Standard reference solutions of 1000μg/ ml, each metal, (traceabletoNIST) were procured from Inorganic Ventures (Christiansburg, VA; USA). Filter paper was used for filtration, where necessary.
Sample treatment
Mild digestion for determination of SO4 2-, PO4?3-in water: Analiquot (50ml) of well-mixed sample was transferred into a conical flask. To this was added 5mL of 6MHCl, and then heated on a hot plate until the volume has been reduced to about 30ml. The digestate was cooled and then filtered through Whatman#1filterpaper. The residue on filter was washed with distilled water. The filtrate was quantitatively transferred to a 50ml volumetric flask, diluted to volume with distilled water, and saved for the determination of sulphate, silica and phosphate.
Pretreatment for determination of NO3 -and NO2 -: A 50ml aliquot of well-mixed sample was filtered through a 0.45μm membrane filter. The filtrate was saved for the determination of nitrate and nitrite. Other sample pre-treatment methods are discussed under individual methods for measurements
Methods for measurements
pH (Electrode method (hach method8156, SM4500H+B. EPA150.1): Procedure: pH was determined on a liquo to fun filtered water samples using a combination pH electrode (IONIXPC-50) multi-parameter test meter. Test results were validated using traceable pH buffer solutions (pH4.01±0.02, 7.00±0.02, 10.0±0.02; Hach Chemicals, Loveland, USA).
Electrical conductivity (direct measurement method (SM2510B, hach method8160): Electrical conductivity was determined by potentiometric method, using a multi-parameter conductivity meter (IONIXPC-50), in the ECmode. Test results were validated against certified conductivity standards (18.0, 1000, and 1990μS/cm; Hach Chemicals, USA)
Procedure: The multi-meter was set to the EC mode and then used for the determination of E Conaliquots of the samples, and LCS.
Total Dissolved Solids (TDS);. (direct measurement method): TDS was determined by potentiometric method, using a multi-parameter meter (IONIXPC-50), in the TDSmode. Test results were validated against certified conductivity standards (18.0,1000, and 1990μS/cm; Hach Chemicals, USA)
Procedure: The multi-meter was set to the (TDS) mode and then used for the determination of TDS on aliquot soft the samples and LCS.
Salinity (direct measurement method):Salinity was determined by potentiometric method, using a multi-parameter conductivity mete rIONIXPC-50), in the salinity mode. Test results were validated against certified salinity standards (0.10, 0.50, 1.0 and 2.0 ppt, Sigma Aldrich, USA)
Procedure: The multi-meter was set to the Salinity mode and then used for the determination of salinity on aliquot soft the samples, and LCS.
Total Suspended Solids (TSS) (gravimetric method (SM2450D; hach method 8158):Analiquot(50-100ml) of the sample was filtered through atarred47mm, 0.45um glass fibre filter. The residue on the filter was dried at 103℃, for 1hour, cooled in adesiccator to 25oC and weighed.
Turbidity (nephelometric method):Procedure: Turbidity of the water sample was measured on a 10mlaliquot, of a homogeneous sample, by ratio turbidimetry of a primary nephelo metric light scatter signal (90°) and transmitted light scatter signal.
Total acidity (buret titration method (SM2320, hach method 8010):Total Acidity of unfiltered samples was determined by buret titration using standard NaOH.
Total alkalinity (buret titration method (SM 2320B, Hach Method 8203):Total Alkalinity of unfiltered samples was determined by buret titration with standard sulphuric acid (0.02N) to colorimetric endpoint, corresponding to H4.3, and includes all forms of alkalinity (carbonate, bicarbonate and hydroxide alkalinities)
Carbon Dioxide (buret titration method):Carbon dioxide of unfiltered samples was determined by buret titration with standard NaOH (0.02M) to colorimetric endpoint, corresponding top H8.3,
Total hardness: Buret titration method (SM 2340C, hach method 8226):Total hardness was determined by the buret titration method. Analiquot (10-50ml) of the test sample was treated with a pH 10 buffer (Hach) and the nitrate with standard 0.08 MEDTA titrant, using calm agite a send-point indicator. The total hardness was calculated as follows:
Dissolved Oxygen (DO): Membrane electrode method (SM 4500G, US EPA 360.1, hach method 8151):Dissolved oxygen was determined by membrane electrode method using a dissolved oxygen meter with Clark- Type amperometry sensor.
Biochemical oxygen demand:5- day BOD Test (APHA 5210- OB) BOD was determined by measuring dissolved oxygen before and after 5 days of incubation of 60ml aliquots of the samples, in the dark at 20oC. A 150ml aliquot of the sample was aerated with an aeration pump. The dissolved oxygen of an aliquot of this oxygenenriched sample was determined (DOinitial). A second aliquot (60ml) was immediately incubated, in a BOD bottle, at 20oC for 5 days in an incubator.
Chemical Oxygen Demand (COD):Closed Reflux, Colorimetric Method (HACH Method 8000, SM 5220C, 5220D)
Phosphate (ascorbic acid method (hach Method 8048, SM 4500-PE):Ortho phosphate reacts with molybdate in an acid medium to produce a mixed phosphate/molybdate complex. Ascorbic acid then reduces the complex to an intense blue color. Measurement is made at 880nm, using as spectrophotometer
Nitrate (cadmium reduction method (hach 8192, SM 4500B, E):Cadmium metal reduces nitrates in the sample to nitrite. The nitrite ion reacts, in acidic medium, with sulfanilic acid to form an intermediate diazonium salt, which couples with chromo tropic acid to form a pink solution, which absorbs at 507nm. Reagents (Hach Chemical Company, USA)
Nitrate standard (0.4mg/LNO3 -N, prepared from 100mg/ LNO3 -N; hach chemicals):To a 15ml aliquot of the prepared sample was added the contents of one NitraVer6 Nitrate Reagent Powder Pillow (Hach). The mixture was shaken and allowed to stand for 5min. To 10ml of the clear solution was added the content of NitriVer3 reagent, and the solution shaken to dissolve the mixture. The mixture was allowed to stand for 15min for complete color development. Thereafter, measurement was made with as spectrophotometer at 507nm.
Nitrite (diazotization method (hach method 10019, EPA method 354.1):Nitrite ion reacts, in acidic medium, with sulfanilic acid to form an intermediate diazonium salt. The salt couples with anchromotropic acid to form a pink solution, which absorbs at 507nm
Chloride (burette titration method (SM4500-ClB, hach method 8225):Chloride was determined by buret titration. Analiquot (10-20ml) of the test sample was titrated with standard silver nitrate titrant, using potassium chromate as end-point indicator.
Sulphate (turbidimetric methods (SM4500E; HACH 8051):Sulphate was determined by turbidimetric method, in which an aliquot of sample filtrate that passed through 0.45μm membrane filter was reacted with barium chloride (HACH Sulfa Ver4 reagent powder). The amount of turbidity in test solution is proportional to sulphate concentration and was determined at 450nm, using as spectrophotometer.
Determination of metals and trace elements by ICP-OES (EPA method 200.7):EPA Method 200.7 is applicable to the analyses of the metals, dissolved and stabilized in aqueous acidic media, and then analyzed by the use of an ICP. The method was used for determination of the specified metals in water, sediment & fish.
Quality Control Sample (QCS):Analysis of a QCS was required for initial and periodic verification of calibration standard solutions, in order to verify instrument performance. The QCS was obtained as Certified Reference Material (CRM) and prepared in the same acid mixture as the calibration standards. The concentration of the analytes in the QCS solution was 1.0mg/L, for each metal
Methods of measurements
Standard multi-element calibration solutions of the metals (1.0-5.0mg/L, each) were prepared for the ICP-OES calibration curves. The solutions were prepared from the stock standard reference solution of the individual metal (1000μg/ml) by appropriated dilution, in a volumetric flask, with deionized water. Except otherwise stated in the procedures, the chemicals used for the analysis of the samples were of analytical grade.
Analytical data for the simultaneous determination of the elements
The metals were determined on the prepared sample solutions by ICP-OES spectrometry. An Agilent SPS-3 Auto sampler was used to deliver the sample solutions to the ICP. A3-second rinse was used to assist with wash out of high concentration of the elements. A reagent blank was determined against a 5-point calibration curve plotted for the standard solutions of metals. Conversion from mg/L of test solution to mg/kg of the sediment or fish sample was obtained from the relationship:
Proximate Analysis
Moisture content
The method described by AOAC (1955) was adopted. The method is based upon removal of water from the sample and its measurements by loss of weight. A clean crucible was weighed and dried in the oven (W1):1.0gof each of the samples was weighed into the crucible (W2) and dried at 105°c for 20 hours. The crucible was then transferred from the oven to desiccators, cooled and reweighed (W3). The percentage moisture content was calculated thus: %Moisture content = 100-(W3-W1/W2 ×100)
Crude protein
Crude protein in the sample fish fillet was quantified following
the procedure of AOAC (1990) by Kjeldahl method. About 2g of
each of the samples were mixed with 10ml of the concentration
H2SO4 in a heating tube. One tablet of selenium catalyst was added
to the tube mixture heated inside a fume cupboard. The digest
was transferred inside distilled water, 10ml of the digest was
mixed with equal volume of 45% NaOH and poured into a Kjeldahl
distillation apparatus. The mixture was distilled and the distillate
collected into 4% boric acid solution containing 3 drops of methyl
red indicator. A total of 50ml distillate was collected and titrated
as well. The sampling was duplicated and the average value taken.
The nitrogen content was calculated and multiplicated with 6.25 to
obtain the crude protein content.
This is given as the percentage Nitrogen=(100×N×14×VF)
T/100×Va
Where: N=Normality of the titrant.
VF=Total volume of the digest=100mlT=Titre value
VA=Aliquot volume distilled
Total lipids (bligh and dryer method)
5-10g wet sample were weighed in pre-weighed 100ml conical flask. 20ml methanol (MeOH) and 10ml chloroform (CHCL3) were added. Samples were homogenized for 2 minutes with an Ultra Turrax. 10ml CHCL3 were added a second time. The mixtures were vigorously mixed for 1minute. About 18ml of distilled water was added (including the water already in the sample). The mixture was vortex again for 1 minute. The two layers were separated by centrifugation for 10minutes at 450g in a thermostatic centrifuge. The lower layers were transferred to a pear-shaped tank with a Pasteur pipette. A second extraction was done with 20ml 10% (v/v) meoh in CHCL3 byvortexting for 2 minutes. The lower CHCL3 phases was added to the first extract. The samples were added to the first extract. The residues were further dried at 104°c for 1 hour
Crude fibre
The method described by AOAC (1995) was used for this
analysis. The 1.0g of the finely ground samples was weighed out
into a round bottom flask, 100ml 0f 1.25% sulphuric acid solution
was added and the mixture was boiled under a reflux for 30
minutes. The insoluble matter was washed several times with hot
water until it was acid free. It was quantitatively transferred into
the flask,100ml of hot 1.25% Sodium Hydroxide (NaOH) solution
was added and the mixture boiled again under reflux for 30minutes
under suction.
The loss in weight of sample on incineration=C1- C2×100
Weight of original sample: % crude fiber =C1 – C2
Total ash
The AOAC (1995) method was used for the determination of total ash content. The procilain crucible was dried in an oven at 100°c for 10minutes. Cooled in desiccators and weighed (W1). About 2g of the sample was placed into the previously weighed crucible and reweighed (W2) and then placed in a furnace for 4 hours at 600°C to ensure proper washing % Ash content =W2-W1×100/W3-W1
Amino acids
Extraction and the instrumentation analyses were carried out by following the modified method (AOAC 2006) in the simultaneous identification and determination of total content of amino acids in food supplements tablets by gas chromatography as described by Obreshkova.
Statistical Analysis
Proximate data were analyzed using graph pad prism. V statistical package was employed in the analysis. Differences were considered significant at (p<0.05). The results were expressed as mean ± SD. The determined differences among treatments were partitioned by the Least Significant Difference (LSD) and the Duncan’s New Multiple Range Test (DNMRT) Duncan.
Results
The mean and the standard deviation of the water parameters and mineral composition are presented in Table 1 above. The Temperature Lagos island (25.15±0.07), Ikorodu (25.2±0.14), Badagry (25.15±0.07), was not significantly different but the pH is however significantly different. Lagos Island (7.42±0.23), Ikorodu (6.03±0.02), Badagry (6.11±0.08). The concentration of dissolved oxygen is highest in Lagos Island with mean value of (4.95±0.35). Arsenic, Cadmium, Lead and Nickel were not detected in the three stations.
The mean concentration of arsenic, cadmium, copper, nickel in the three sampling stations showed no significant difference (p>0.05). The percentage concentration of zinc and iron was found to be statistically difference (p<0.05) The percentage mineral composition of zinc and iron were found to be statistically different (p<0.05), while arsenic, cadmium, copper, lead, nickel showed no significant difference (p>0.05).
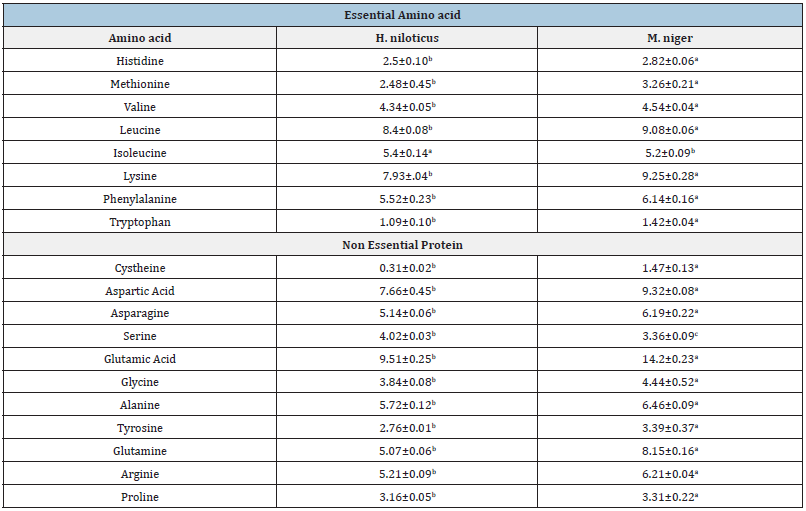
Badagry has the lowest concentrate in Arsenic with value of (0.06±0.01), while Lagos Island has highest concentrate in Arsenic (0.11±0.04). The zinc content generally ranged from a minimum of in Lagos Island, (415.5±40.45) and maximum of (1826.35±63.29) in Ikorodu. The moisture content of M. niger has the highest concentration (77.61±0.60) while H. niloticus has mean value of (55.68±3.87). The highest concentration of protein was observed in H. niloticus (21.33±1.15) while the lowest was in M. niger. There were significant differences in all the samples (p<0.05). H. niloticus has the highest concentrate of isoleucine in the table above of (5.4±0.14). M. niger has the highest concentrate of essential amino acid except in histidine and isoleucine. All the sampling species were all statistically different (p<0.05). M. niger has the highest value of glutamine acid of (8.15±0.16). The result above shows that M. niger has the highest concentrate of non-essential amino acid except in serine. All the sampling species were all statistically different (p<0.05).
Discussion
The physico-chemical parameters of the three sampling stations; Ikorodu, Badagry and Lagos island waters investigated are presented in the (Table 1) above. There were variations in the physical and chemical characteristics of the sampled water used for test. The reported values referred to the mean values of the water qualities concentration of the minerals, proximate analysis, amino acids of sampled stations (Ikorodu, Badagry and Lagos island), Lagos State, Nigeria, Physical and chemical characteristics, such as temperature, Ikorodu (25.2±0.14), Badagry (25.15±0.07) and Lagos island (25.15±0.07) which met with (WHO 2011) regulatory limit. pH value of Ikorodu (6.03±0.02) and Badagry (6.11±0.08) was within the (WHO 2011) regulatory limit in which Lagos island (7.42±0.23) water sample was slightly alkaline exceeding the regulatory limit. The alkaline pH of Lagos island used in this study could have resulted from decaying organic matter Rim-Rukeh, Ikhifa, and Okokoyo; Adeogun, Chukwuka, and Ibor. Virtually all parameters analyzed deviated from the WHO standard values of 2011. Electrical conductivity, magnesium, chloride exceed the WHO permissible limit, and may be attributed to high level anthropogenic activities around the sampling stations respectively. Mineral element analyzed in Table 1, copper was not detected in Lagos Island, 0.01mg/l was detected in Ikorodu and Badagry (0.02mg/l) which met with the WHO standard. Copper is essential for good health, but high intakes can cause health problems such as liver and kidney damage [20,21]. Iron was largest in Ikorodu (0.31mg/l). Fish is a major source of iron for adult and children and iron deficiency causes anemia [21]. The level of magnesium and chloride is higher than WHO standard limit.
Table 1:Water parameters and mineral compositions of Sample locations
Means with different superscript for a given parameter in the same role are significantly different (p<0.05)
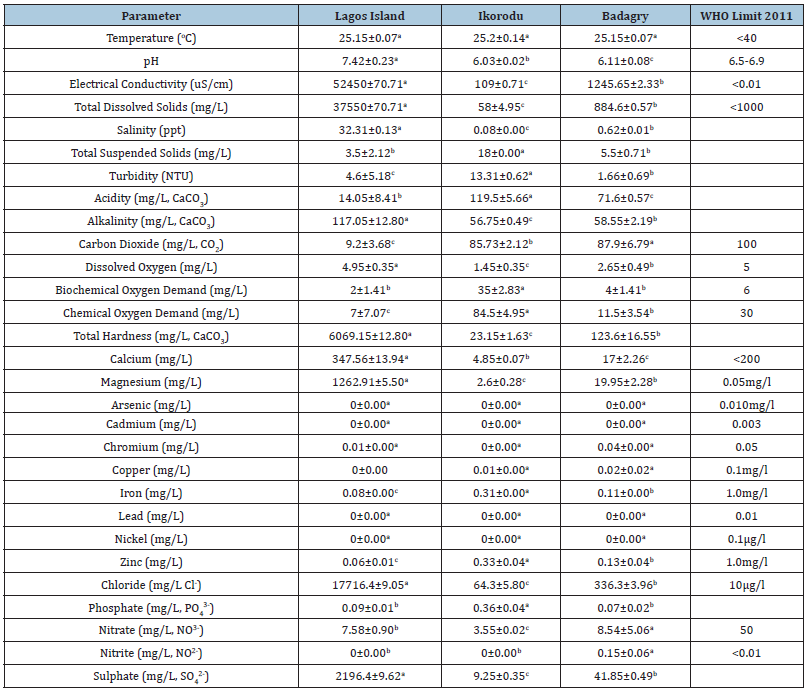
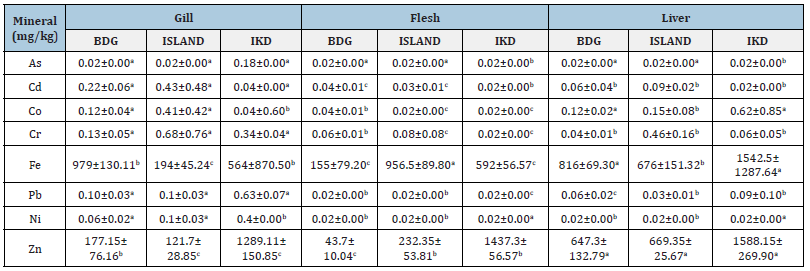
Table 2:Mineral composition in the gills, flesh and liver of Heterotis niloticus in Badagry,Lagos Island and Ikorodu
sample stations.
The means with different super script for a given parameter in the same role are significantly different (p<0.05).
Conclusion
Table 3:Mineral composition in the gills, flesh and liver of Macolor niger in Badagry, Lagos Island and Ikorodu sample
stations.
Means with different superscript of a given parameter in the same role are significantly difference (p<0.05).
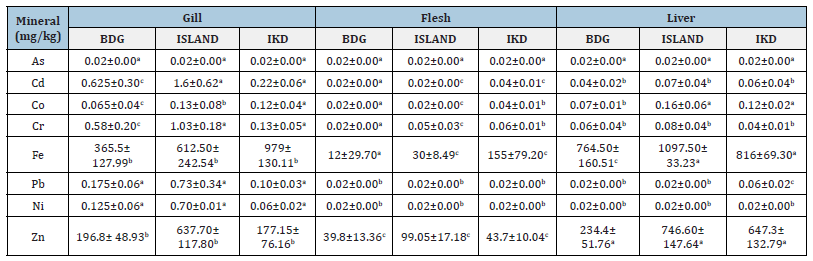
Table 4:Concentration of Sediments in the Study locations.
The means with different superscript for a given parameter in the same role are significantly different (p<0.05).
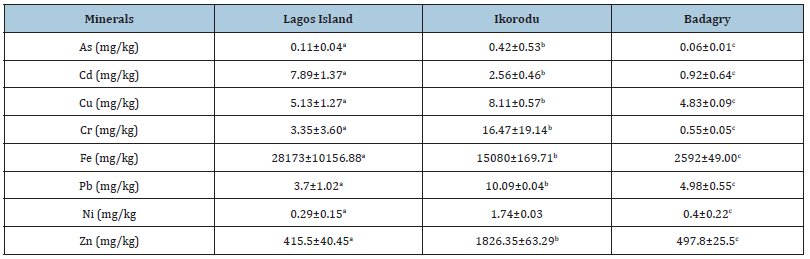
Table 5:Proximate Analysis of Sampled Fish.
Means with different superscript for a given parameter in the same role are significantly different (p<0.05)
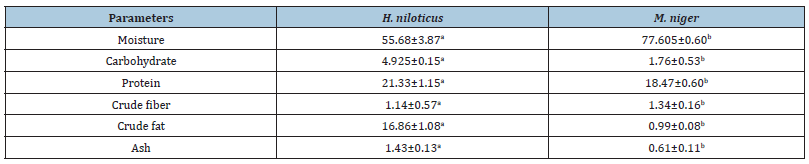
Table 5:Proximate Analysis of Sampled Fish.
Means with different superscript for a given parameter in the same role are significantly different (p<0.05)

Table 6&7:Essential and Non-essential amino acid composition in sampled fish.
Means with different superscript for a given parameter in the same role are significantly different (p<0.05).
Conflict of Interests
The authors declare that there is no conflict of interests.
References
- Cobos Á, Díaz O (2015) Chemical composition of meat and meat products. In: Cheung P (edn), Handbook of Food Chemistry. Springer, Germany.
- Hammed AM, Awe FA, Amosu AO, Ibrahim UB (2020) Evaluation of the nutritional composition (proximate, mineral and amino acids) of tilapinis (tilapia zillii and sarotherodon galilaeus) in badagry creek, Lagos, Nigeria. Research Gate 5(1): 71-78.
- Fawole OO, Ogundiran MA, Ayandiran TA, Olagunju OF (2007) Mineral composition in some selected freshwater fishes in Nigeria. J Food Safety 9: 52-55.
- Antony PJP (2015) Minerals in fish: Does the source matter? Wageningen University, Netherlands, pp. 267.
- (2011) NRC: Nutrient Requirements of Fish and Shrimp. The National academies Press, Washington, USA.
- Jaishankar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN (2014) Toxicity, mechanism and health effects of some heavy metals. Interdiscip toxicol 7(2): 60-72.
- FAO (2020) The state of world fisheries and aquaculture. Sustainability in action, Rome, Italy.
- Lall SP, Kaushik SJ (2021) Nutrition and metabolism of minerals in fish. Animals (Basel) 11(9): 2711.
- Antony PJP, Fjelldal PG, Remø SC, Selvam CH, Hamre K, et al. (2022) Dietary electrolyte balance of Atlantic salmon (salmo salar) freshwater feeds: Impact on osmoregulation, mineral metabolism and performance in seawater. Aquaculture 546: 737305.
- Lall SP (2002) The minerals. In: John EH, Ronald WH (eds.), Fish Nutrition, (3rd edn), Academic Press, San Diego, California, USA, pp. 259-308.
- Rajeshkumar S, Li X (2018) Bioaccumulation of heavy metals in fish species from the meiliang Bay, taihu Lake, China. Toxicology Reports 5: 288-295.
- Elsayed YM, Abdel-Warith AWA, Al-Asgah NA, Elthebite SA, Mostafizur Rahman M (2021) Nutritional value and bioaccumulation of heavy metals in muscle tissues of five commercially important marine fish species from the red sea. Saudi Journal Biol Sci 28(3): 1860-1866.
- Lilly TT, Immaculate JK, Jamila P (2017) Macro and micronutrients of selected marine fishes in tuticorin, Southeast coast of India. International Food Research Journal 24(1): 191-201.
- Kiczorowska B, Samolińska W, Grela ER, Bik-Małodzińska M (2019) Nutrient and mineral profile of chosen fresh and smoked fish. Nutrients 11(7): 1448.
- Kaushik SJ, Seiliez I (2010) Protein and amino acid nutrition and metabolism in fish: Current knowledge and future needs: Protein and amino acid nutrition and metabolism in fish: current knowledge and future needs. Aquaculture Research 41(3): 322-332.
- Espe MA, Lemme A, Petri A, El-Mowafi A (2006) Can Atlantic salmon (Salmo salar) grow on diets devoid of fish meal? Aquaculture 255(1-4): 255-262.
- Wu G (2010) Functional amino acids in growth, reproduction, and health. Adv Nutr 1(1): 31-7.
- Falco F, Stincone P, Cammarata M, Brandelli A (2020) Amino acids as the main energy source in fish tissues. Aquaculture and Fisheries Studies 3(1): 1-11.
- Li X, Zheng S, Wu G (2020) Nutrition and metabolism of glutamate and glutamine in fish. Amino Acids 52(5): 671-691.
- Boyd CE, Davis JA (1978) Concentration of selected element and ash in Bluegill (Lepomis macrochirus) and certain other freshwater fish. Trans Am Fish Soc 107(6): 862-867.
- Demirezen D, Uruc K (2006) Comparative study of trace element in certain fish, meat and meat products. Meat Science 74: 255-260.
- Adewoye SO, Omotosho JS (1997) Nutrient composition of some freshwater fishes in Nigeria. Biosci Res Commun 11(4): 333-336.
- Briffa J, Sinagra E, Blundell R (2020) Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 6(9): e04691.
- Abe H, Okuma E (1991) Effect of temperature on the buffering capacities of histidine related compounds and fish skeletal muscle. Nippon Suisan Gakkaishi 57(11): 2101-2107.
- Abe H (2000) Role of histidine-related compounds as intracellular proton buffering constituents in vertebrate muscle. Biochemistry(Moscow) 65(7): 757-765.
- Andersen SM, Holen E, Aksnes A, Ronnestad I, Zerrahn JE, et al. (2015) Adult Atlantic salmon (Salmo salar L.) adapts to long-term surplus dietary arginine supplementation. Aquaculture Nutrition 21(3): 355-363.
- Anderson PM (1981) Purification and properties of the glutamine-dependent and n-acetyl-lglutamate-dependent carbamoyl phosphate synthetase from liver of squalus-acanthias. Journal of Biological Chemistry 256(23): 2228-2238
- AOAC (2005) Official method of analysis (18th edn), Association of official analytical, Washington, USA.
- Baker DH (1986) Problems and pitfalls in animal experiments designed to establish dietary requirements for essential nutrients. J Nutr 116: 2339-2349.
- Breck O, Bjerkås E, Campbell P, Rhodes JD, Sanderson J (2005) Histidine nutrition and genotype affect cataract development in Atlantic salmon, Salmo salar L. Journal of Fish Diseases 28(6): 357-371.
- Breck O, Bjerkas E, Campbell P, Arnesen P, Haldorsen P, et al. (2003) Cataract preventative role of mammalian blood meal, histidine, iron and zinc in diets for Atlantic salmon (Salmo salar L.) of different strains. Aquaculture Nutrition 9(5): 341-350.
- Broadhurst CL, Wang Y, Crawford MA, Cunnane SC, Parkington JE, et al. (2002) Brain-specific lipids from marine, lacustrine, or terrestrial food resources: Potential impact on early African Homo sapiens. Comp Biochem Physiol B Biochem Mol Biol 131(4): 653-673.
- Buentello JA, Gatlin DM (2001) Plasma citrulline and arginine kinetics in juvenile channel catfish, Ictalurus punctatus, given oral gabaculine. Fish Physiology and Biochemistry 24(2): 105-112.
- Buentello JA, Gatlin DM (2000) The dietary arginine requirement of channel catfish (Ictalurus punctatus) is influenced by endogenous synthesis of arginine from glutamic acid. Aquaculture 188(3-4): 311-321.
- Bury NR, Walker PA, Glover CN (2003) Nutritive metal uptake in teleost fish. J Exp Biol 206(Pt 1): 11-23.
- Carpene E, Martin B, Libera LD (1998) Biochemical differences in lateral muscle of wild and farmed gilthead sea bream (Sparus aurata L.). Fish Physiol Biochem 19: 229-238.
- AOAC (2005) Official method of analysis (18th edn). Association of official analytical chemists Washington DC, USA, pp. 106.
- Cho C, Kaushik S (1990) Nutritional energetics in fish: Energy and protein utilization in rainbow trout (Salmo gairdneri). World Rev Nutr Diet 61: 132-172.
- Clements S, Schreck CB (2004) Evidence that GABA mediates dopaminergic and Serotonergic pathways associated with locomotor activity in juvenile chinook salmon (Oncorhynchus tshawytscha). Behav Neurosci 118(1): 191-198.
- Cooper CA, Bury NR, Grosell M (2006) The effects of pH and the Iron redox state on Iron uptake in the intestine of a marine teleost fish, gulf toadfish (Opsanus beta). Comp Biochem Physiol A Mol Integr Physiol 143(3): 292-298
- Crawford M, Bloom M, Broadhurst C, Schmidt W, Cunnane S, et al. (1999) Evidence for the unique function of docosahexaenoic acid during the evolution of the modern hominid brain. Lipids 34(1): S39-S47.
- Cunnane S, Stewart K (2010) Human brain evolution: The influence of freshwater and marine food resources. Biological Anthropology pp: 232.
- Dabrowski KR (1985) Energy budget of coregonid (Coregonus spp.) fish growth, metabolism and reproduction 45(3): 358-364.
- Davis DA, Gatlin DM (1996) Dietary mineral requirements of fish and marine crustaceans. Reviews in Fisheries Science 4(1).
- Eddy FB (2005) Role of nitric oxide in larval and juvenile fish. Comparative Biochemistry and Physiology a-Molecular & Integrative Physiology 142(2): 221-230.
- FAO (2012) Demand and supply of aquafeed and feed ingredients for farmed fish and crustaceans: Trends and future prospects. In State of World Fisheries and Aquaculture Rome, Italy.
- Jiang J, Zheng T, Zhou XQ, Liu Y, Feng L (2009) Influence of glutamine and vitamin E on growth and antioxidant capacity of fish enterocytes. Aquaculture Nutrition 15(4): 409-414.
- Korte JJ, Salo WL, Cabrera VM, Wright PA, Felskie AK , et al. (1997) Expression of carbamoyl-phosphate synthetase III mRNA during the early stages of development and in muscle of adult rainbow trout (Oncorhynchus mykiss). J Biol Chem 272(10): 6270-6277.
- Kaushik S (2002) Mineral nutrition. In: Guillaume J, Kaushik S, Bergot P, Metailler R (eds.), In Nutrition and Feeding of Fish and Crustaceans, Springer-Praxis Publishing Ltd, UK, pp. 169-181.
- Lall S, Milley JE (2008) Trace mineral requirements of fish and crustaceans. Trace elements in animal production systems 203.
- Lall SP (1995) Macro and trace elements in fish and shellfish. In: Ruiter A (Ed.), Fish and fishery products. CAB International, Tunisia, pp: 187-213.
- Mori M (2007) Regulation of nitric oxide synthesis and apoptosis by arginase and arginine recycling. J Nutr 137(6 Suppl 2): 1616S-1620S.
- Mitchell HH (1962) In comparative nutrition of man and domestic animals. (1st edn), Academic Press, USA.
- Nestel PJN (2000) Fish oil and cardiovascular disease: Lipids and arterial function. Am J Clin Nutr 71(1 Suppl): 228S-231S.
- (1993) NRC: Nutrient Requirement of Fish. National Academy Press, Washington, USA.
- Pernow J, Jung C (2013) Arginase as a potential target in the treatment of cardiovascular disease: reversal of arginine steal? Cardiovascular Research 98(3): 334-343.
- Pfeffer E, Pieper A (1979) Application of factorial approach for deriving nutrient requirements of rainbow trout. In: JE Halver and KT Tlews (eds.), Fish nutrition and fish feed technology, Berlin, Germany, 2: 545-553.
- Pfeffer E, Potthast V (1977) Studies on the use of energy, protein and mineral elements in growth of rainbow trout. Fortschr Tierphysiol. Tierernahr 8: 32-5.
- Portugal TR, Aksnes A (1983) Arginase activity in different fish species and tissues. Comparative Biochemistry and Physiology B-Biochemistry & Molecular Biology 76(1): 15-16.
- Trudeau VL, Spanswick D, Fraser EJ, Lariviere K, Crump D, et al. (2000) The role of amino acid neurotransmitters in the regulation of pituitary gonadotropin release in fish. Biochem cell Biol 78(3): 241-259.
- Walker SP, Keast D, McBride S (1996) Distribution of glutamine synthetase in the snapper (Pagrus auratus) and implications for the immune system. Fish Physiol Biochem 15(3): 187-194.
- Rhodes JD, Breck O, Waagbo R, Bjerkas E, Sanderson J (2010) N-Acetylhistidine, a novel osmolyte in the lens of Atlantic salmon (salmo salar l.). Am J Physiol Regul Integr Comp Physiol 299(4): R1075-1081.
- Schwarz FJ (1995) Determination of mineral requirements of fish. Journal of Applied Ichthyology 11(3-4): 164-174.
- Shearer KD (1995) The use of factorial modeling to determine the dietary requirements for essential elements in fishes. Aquaculture 133(1): 57-72.
- Tacon AGJ, Silva SSD (1983) Mineral composition of some commercial fish feeds available in Europe. Aquaculture 31(1): 11-20.
- Watanabe T, Kiron V, Satoh S (1997) Trace minerals in fish nutrition. Aquaculture 151(1-4): 185-207.
- Wu GY, Bazer FW, Davis TA, Kim SW, Li P, et al. (2009) Arginine metabolism and nutrition in growth, health and disease. Amino Acids 37(1): 153-168.
© 2022 Ayofe M Hammed. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






