- Submissions

Full Text
Novel Research in Sciences
Toxicity Assessment of Biosynthesized Iron Oxide Nanoparticles Using Casia Fistula Leaf Extract, Aggregation in Gills and Biochemical Parameters of Platy Xiphophorus Maculatus
Muthuswami Ruby Rajan1*, Jahir Hussain Shabnam1 and Dhandapani Suganya2
1Department of Biology, The Gandhigram Rural Institute-Deemed to be University, India
2Muthaiyammal College of Arts and Science, Rasipuram, Namakkal-647 408, Tamil Nadu, India
*Corresponding author: Muthuswami Ruby Rajan, Department of Biology, The Gandhigram Rural Institute-Deemed to be University, Gandhigram-624 302, Tamil Nadu, India
Submission: June 06, 2022;Published: June 16, 2022
.jpg)
Volume11 Issue3June, 2022
Abstract
The present study aimed at the toxicity of biosynthesized Iron oxide nanoparticles using Casia fistula leaf extract, aggregation in gills and biochemical parameters of Platy Xiphophorus maculatus. Casia fistula leaf extract was used for the synthesis of iron oxide nanoparticles and described using UVVisible spectrophotometer, Scanning Electron Microscope, Energy Dispersive X-Ray Spectroscopy, Fourier Transform Infrared Spectroscopy and X-Ray Diffraction. Aggregation of biosynthesized iron oxide nanoparticles in the gills and biochemical parameters were estimated by exposing platy for 7 days. UV-VIS image of iron oxide nanoparticles was observed at 371.79nm. SEM image was observed at the wavelength range 5 and 10mm. EDX spectrum shows Fe and O elements and located between 0.5 to 6.5KeV. FT-IR spectrum was ascertained in the line of 4000-400cm-1. XRD results confirmed the crystal expression, and the moderate size is 20±24nm. Aggregation of iron oxide nanoparticles in gills increased and biochemical parameters such as protein, carbohydrate and lipids in muscle and gill of platy were gradually decreased with increased concentration of iron oxide nanoparticles. The present study concluded that the biosynthesized iron oxide nanoparticles using Casia fistula leaf extract affect the biochemical parameters of platy.
Keywords: Keywords: Toxicity; Casia fistula; Biosynthesis; Iron oxide nanoparticles; Aggregation; Biochemical; Platy fish
Introduction
Many researchers have discovered many plant species and components that contain antioxidant compounds such as nitrogenous base amino acids, polyphenols and sugars [1]. These compounds perform the function of a capping agent for the synthesis of nanoparticles [2]. Generally, metal nanoparticles are manufactured in two ways such as bottom to up and top to bottom. Metal and metal oxide nanoparticles om plant extract are generally stable even after 1 month and do not undergo any change [3]. Green synthesis of various metallic nanoparticles has re-evaluated plants for their natural ability to reduce toxic and hazardous chemicals. Nanoparticles of plant-related parts such as leaves, stems flowers, bark, roots, seeds and their metabolites have been used for biosynthesis [4]. The synthesis of iron oxide nanoparticles depends upon plant type and phytochemical concentration, whereas microorganisms and other methods require more time to reduce ions to nanoparticles [5]. The main advantage of biosynthesis is to control the particle size, shape and physicochemical properties. Also, biomolecules of plants can act as capping and reducing agents and it increases the percentage of reduction rate and stabilization of nanoparticles [6]. The different organic and inorganic materials such as carbohydrates, phlorotannin’s, anthraquinones, phenolic compounds, alkaloids, flavonoids, glycosides, saponins, tannins, sterols, amino acids, terpenoids and quinines are present in the extract can act as powerful reducing agents. Among the phytochemicals, phenolic compounds and flavonoids play a vital role in the formation of iron oxide nanoparticles [7]. Nanoparticles released into the water ecosystem affect the organisms including fishes [8]. The present work aimed at the influence of biosynthesized iron oxide nanoparticles using Casia fistula leaf extract, aggregation in gills and biochemical parameters of platy Xiphophorus maculatus.
Materials and Methods
For the experimental study, iron oxide nanoparticles (Fe3O4) were biosynthesized by using the leaf of Casia fistula as a reducing agent. Fresh leaves were collected from The Gandhigram Rural Institute- Deemed to be a university campus, Gandhigram, Tamil Nadu, India, surface cleaned with running water to remove debris and other contaminated organic contents and dried at room temperature. The powdered leaf was placed in the Soxhlet apparatus, added 300ml of methanol and run up to 3 hours, the extract was collected in the round bottom flask. For the synthesis of iron oxide nanoparticles, Casia fistula leaf extract was used as a capping agent. For toxicity studies, Platy Xiphophorus maculatus fingerlings (3.5±1.5g) were collected from A.M Fish Farm, Madurai, Tamil Nadu, India and transported to the laboratory and acclimated in round plastic troughs for 15 days at 28±2 °C. During acclimation, fishes were fed with trainee feed containing fish meal, groundnut oil cake, wheat flour and rice bran in the form of dry Pellets. The synthesized nanoparticles were described using UV-VIS Spectrophotometer, Scanning Electron Microscopy, Energy Dispersive X-ray Spectrometer Fourier Transform Infrared Spectroscopy and X-Ray Diffraction. Water quality parameters such as pH, Temperature, dissolved oxygen, dissolved carbon dioxide, hardness, alkalinity, and Chloride were estimated [9]. For assessment of iron oxide nanoparticles toxicity, different concentrations (0, 10, 100, 500, 750 and 1000ppm) of the iron oxide nanoparticles were taken. Control was maintained with tap water and without Iron oxide nanoparticles. 5 fish with an average weight of 4-5g were introduced and the behaviour and survival were observed in each concentration for 96hrs. For sub-lethal studies, platy was exposed to different concentrations (0, 100, 200, 300 and 400ppm) for 7 days and iron aggregation in the gills and biochemical parameters such as protein, carbohydrate, and lipid [10-12] in the muscle, and gill were estimated. The fish used is as per the Institutional Ethical Committee for Research on Human and Animal subjects.
Results and Discussion
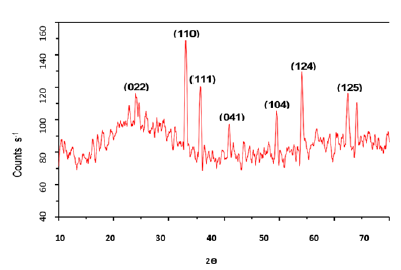
The absorbance spectra of Fe3O4 nanoparticles were measured at 400nm (Figure 1). In the synthesized iron oxide nanoparticles maximum absorption peak is 371.79nm. Pattanayak & Nayak et al. [13,14] reported the absorption peak at 216-265nm from various plant extracts and sorghum bran extract respectively. Sneha Shah et al. [15] reported the maximum UV-Vis absorbance peak at 190nm from the stem extracts of Euphorbia milii, 202nm for Tridax procumbens and flower extracts used by the synthesis of iron oxide nanoparticles and approximate crystal size at 195nm for Euphorbia milii and 194nm for Tinospora cordifolia. Scanning Electron Microscopy indicates the spherical and rectangular shape of synthesized nanoparticles (Figures 2a & 2b). Green synthesized of iron oxide nanoparticles were spherical in [16]. The element composition shows the peaks are located between the 0.5KeV and 6.5KeV (Figure 3). Also reported that the elemental composition of iron oxide nanoparticles was Iron, Oxygen, Calcium and Chloride [17]. The FTIR bands observed at 3437.05, 2918.88, 1714.61, 1628.25 and 543.93 are associated with functional groups of alcohol, phenols, primary amines, ketones and O-H, C-H, C-Br stretching of proteins (Figure 4). The XRD diffraction peaks are indexed as 10.75° (020), 24.12° (022), 33.14° (110), 35.62° (111), 40.87° (041), 49.47° (104), 54.02° (124) and 62.45° (125) (Figure 5). Similar reports were reported by the presence of Fe3O4- nanoparticles on the surface of rice straw evidenced by the absorption bands at around 295-541cm-1 that confirm the Fe‒O stretch [18,19]. From the XRD the average size of the crystal is 20±14. Wu et al. [20,21] studied that the Fe3O4 nanoparticle synthesis followed by the co-precipitation method and its average particle size was calculated at 19.4nm and 11.4nm. Crystallite size of synthesized Fe3O4 nanoparticle was 16.79nm and range of 17– 25nm by using leaf extract of seaweed Kappaphycus alvarezii and brown seaweed (Sargassum muticum) extract [22,23].
Figure 1: UV-VIS image of iron oxide nanoparticles.
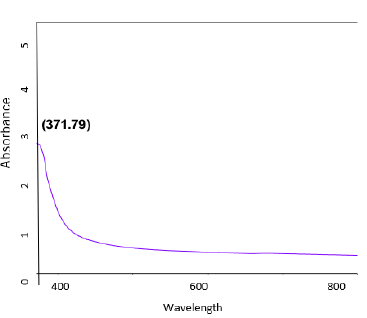
Figure 2: SEM image of iron oxide nanoparticles (a-5μm; b-10μm).
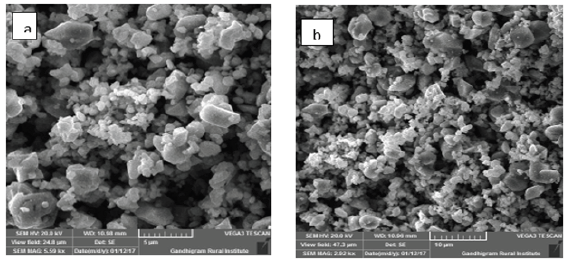
Figure 3: EDAX image of iron oxide nanoparticles.
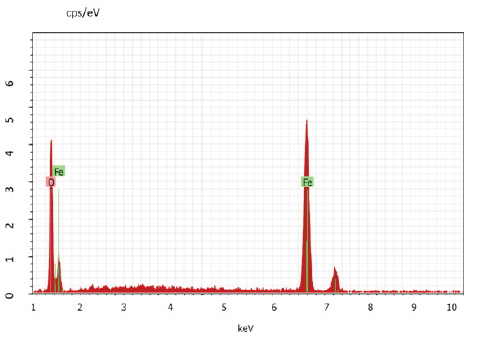
Figure 4: FT-IR image of iron oxide nanoparticles.
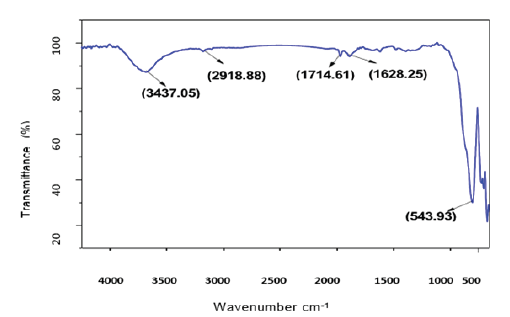
Figure 5: XRD image of iron oxide nanoparticles.
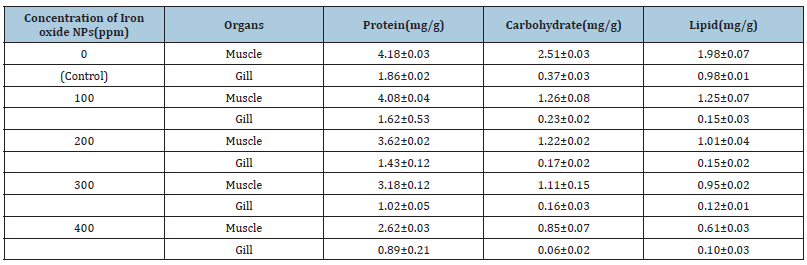
Water quality parameters and behaviour of the fish were presented in Tables 1 & 2. Similarly, Hao et al. [24] reported that the Physico-chemical parameters of water samples are pH-7.1, dissolved oxygen - 6.5mg/L, temperature- 22 ºC, and total hardness- 77.7mg/L. Shahzad et al. [25] reported that the mean values of physicochemical parameters of water samples such as temperature, pH, Dissolved Oxygen (DO), total alkalinity and total hardness are 27 ºC, 7.7, 7.00mg/L, 202mg/L and 51.6mg/L, respectively were similar to the present study. Khunyakari et al. [26] reported toxicity of nickel, copper and zinc in Poecilia reticulata that caused raised secretion like mucus over gills, excessive excretion, anorexia and inflated fin movement. Survival studies of Platy Fish exposed to Iron oxide Nanoparticles are presented in Table 3. The mortality/ survival of fish in iron oxide treated was recorded after 96 hours and the concentration at which 25% mortality of fish occurred was taken as the median lethal concentration [27]. 100% mortality was observed in 6 hours, under the influence of different concentrations of iron oxidnanoparticles and the toxic effect was observed on haematological parameters. Iron aggregation in platyfish gills was gradually increased (Table 4). The excessive aggregation of free iron can cause toxicity generation of reactive oxygen species and induction of cell death The transport of the iron in the blood is performed by plasma protein transferrin and can aggregate in the case of the iron overload in zebrafish. The increased iron concentration on gills is due to hydrolysis and polymerization in agreement with the fish body [28]. Protein, carbohydrate and lipid in muscle and gill decreased with increased concentration of iron oxide nanoparticles (Table 5). The biochemical mechanisms in an organism play an important role during stress conditions due to the presence of toxicants in the aquatic ecosystem. Yesudass Thangam [29] reported that pollutant in aquatic media causes its affect fishes at the cellular or molecular level which results in significant changes in biochemical parameters.
Table 1:Water quality parameters of tap water.
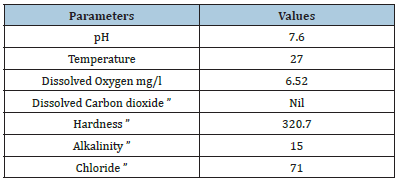
Table 2:Basic observation of platy fish exposed to iron oxide nanoparticles.
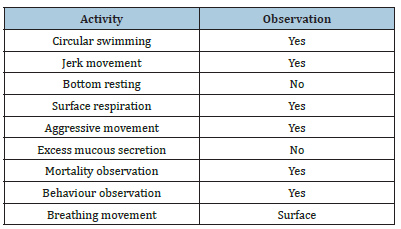
Table 3:Survival Studies of platy Fish exposed to iron oxide nanoparticles.
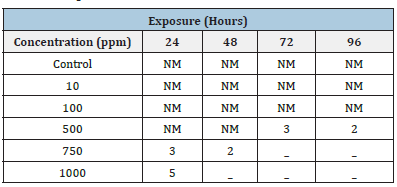
Table 4:Accumulation of iron oxide nanoparticles in gills of platy fish.
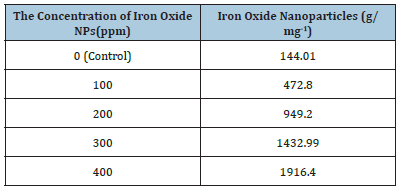
Table 5:Biochemical parameters of platy exposed to different concentrations of iron oxide nanoparticles.
Conclusion
Iron oxide nanoparticles were biosynthesized using Casia fistula leaf extract and characterized using UV-Vis, SEM, EDAX, FTIR and XRD. As the quantity of Iron oxide nanoparticles increased the concentration in the gill of platy was also increased. Decreased protein, carbohydrate and lipid content was observed in muscle and gill of platy.
Acknowledgement
The authors thank the Dept. of Biology, GRI, Gandhigram, India for executing this work.
References
- Vanathi P, Rajiv P, Narendran S, Rajeshwari S, Rahman PK, et al. (2014) Biosynthesis and characterization of phyto mediated zinc oxide nanoparticles: A green chemisty approach. Materials Letters 134: 13-15.
- Zayed MF, Eisa WH, Shabaka AA (2012) Malva Passiflora extract assisted green synthesis of silver nanoparticles. Spectrochimica Act A: Molecular and Biomolecular Spectroscopy 98: 423-428.
- Huang L, Weng X, Chen Z, Megharaj M, Naidu R (2014) Synthesis of iron-based nanoparticles using oolong tea extract for the degradation of malachite green. Spectrochimica Acta A: Molecular and Biomolecular Spectroscopy 117: 801-804.
- Reddy NJ, Vali DN, Rani M, Rani SS (2014) Evaluation of antioxidant, antibacterial and cytotoxic effects of green synthesized silver nanoparticles by Piper longum Materials Science and Engineering C Mater Biol Appl 34: 115-122.
- Rai M, Yadav A, Gade A (2008) CRC 675 - current trends in phytosynthesis of metal nanoparticles. Critical Reviews in Biotechnology 28(4): 277-284.
- Latha N, Gowri M (2014) Bio Synthesis and characterization of Fe3O4 nanoparticles using Caricaya papaya leaves extract. Inernational Journal of Scientific Research 3(11): 1551-1556.
- Aromal SA, Philip D (2012) Green synthesis of gold nanoparticles using Trigonella foenum-graecum and its size-dependen catalytic activity. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 97: 1-5.
- Farre M, Gajda Schrantz K, Kantiani L, Baecelob D (2009) Ecotoxicity and analysis of nanomaterials in the aquatic Anal Bioanalyt Chemistry 393(1): 81-95.
- APHA, AWWA and WEF (2012) Standard methods for the examination of water and wastewater. (22nd edn). In: Rice EW, Baird RB, Eaton AD, and Clesceri LS (Eds.), Washington, D.C. American Public Health Association, USA.
- Lowry OH, Rosenbrough NJ, Farr AL (1951) Protein measurement with the folin phenol reagent. Journal of Biology and Chemistry 193(1): 265-275.
- Carrol NV, Longley RW, Roe JH (1956) The determination of glycogen in liver and muscle by use of anthrone reagent. Journal of Biology and Chemistry 220(2): 583-593.
- Barnes H, Blackstock J (1973) Estimation of lipids in marine animals and tissues detailed investigation of the sulphophosphovanillin method for total lipids. Journal of Experimental Morphology, Biology and Ecology 12: 103-118.
- Pattanayak M, Nayak PL (2013) Eco friendly green synthesis of iron nanoparticles from various plants and spices extract. International Journal of Plant, Animal and Environmental Sciences 3(1): 68-78.
- Njagi EC, Huang H, Stafford L, Genuino H, Galindo HM, et al. (2011) Biosynthesis of iron and silver nanoparticles at room temperature using aqueous sorghum bran extracts. Langmuir 27(1): 264-271.
- Sneha S, Dasgupta S, Mousumi C, Raji V, Murtaza H (2014) Green synthesis of iron nanoparticles using plant extracts. International Journal of Biological and Pharmaceutical Research 5(6): 549-552.
- Eshaghi Z, Vafaeinezhad F, Hooshmand S (2016) Green synthesis of magnetic iron nanoparticles coated by olive oil and verifying its efficiency in extraction of nickel from environmental samples via UV-Vis spectrophotometry. Process Safety and Environmental Protection 102: 403-409.
- Eric C, Hui H, Lisa S, Homer G, Hugo M, et al. (2011) Biosynthesis of iron and silver nanoparticles at room temperature using aqueous sorghum bran Langmuir 27(1): 264-271.
- Khandanlou R, Ahmad MB, Shameli K, Kalantari K (2013) Synthesis and characterization of rice straw/Fe3O4 nanocomposites by a quick precipitation method. Molecules 18(6): 6597-6607.
- Karaoglu E, Baykal A, Erdemi H, Alpsoy L, Sozeri H (2011) Synthesis and characterization of DL-thioctic acid (DLTA) - Fe3O4 Journal of Alloys and Compounds 509(37): 9218-9225.
- Wu S, Aizhi S, Zhai F, Wang J, Xu W, et al. (2011) Fe3O4 magnetic nanoparticles synthesis from tailings by ultrasonic chemical co- precipitation. Materials Letters 65(12): 1882-1884.
- Yuanbi Z, Zumin Q, Jiaying H (2008) Preparation and analysis of Fe3O4 magnetic nanoparticles used as targeted-drug carriers. Chinese Journal of Chemical Engineering 16(3): 451-455.
- Yew YP, Shameli K, Miyake M, Kuwano N, Ahmad Khairudin NBB, et al. (2016) Green synthesis of magnetite (Fe3O4) nanoparticles using seaweed (Kappaphycus alvarezii) extract. Nanoscale Research Letters 11(1): 1-7.
- Mahdavi M, Namvar F, Ahmad MB, Mohamad R (2013) Green biosynthesis and characterisation of magnetic iron oxide (Fe3O4) nanoparticles using seaweed (Sargassum muticum) aqueous extract. Molecules 18(5): 5954-5964.
- Hao L, Chen L, Hao J, Zhong N (2013) Bioaccumulation and sub-acute toxicity of zinc oxide nanoparticles in juvenile carp (Cyprinus carpio): A comparative study with its bulk counterparts. Ecotoxicology and Environmental Safety 91: 52-60.
- Shahzad K, Naeem KM, Jabeen F, Kosour N, Shakoor CA, et al. (2018) Toxicity of zinc oxide nanoparticles (ZnO NPs) in tilapia (Oreochromis mossambicus): tissue accumulation, oxidative stress, histopathology and genotoxicity. International Journal of Environmental Science and Pollution 25(16): 15943-15953.
- Khunyakari RP, Vrushali T, Sharma RN, Tare V (2001) Effects of some trace heavy metals on Poeciliareticulata. Journal of Environmental Biology 22(2): 141-144.
- Thorp VJ, Lake PS (1974) Toxicity bioassays of cadmium on selected freshwater invertebrates and the interaction of cadmium and zinc on the freshwater shrimp, Paratya tasmaniensis Australian Journal of Marine and Water Research 25: 97-104.
- Teien HC, Garmo OA, Atland A, Salbu B (2008) Transformation of iron species in mixing zones and accumulation on fish gills. Environmental Science and Technology 42(5): 1780-1786.
- Yesudass T (2014) Effect of nitrite toxicity in carbohydrate metabolism to fresh water fish Cirrhinus Mrigala. IOSR Journal of Pharmacy and Biological Science 9(5): 03-11.
© 2022 Muthuswami Ruby Rajan. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






