- Submissions

Full Text
Novel Research in Sciences
The Assembly in Synthetic DNA (E. coli) Crown Cells with Inorganic Salts and the Transformation of DNA Crown Cells to Crystal-Like Substances in the Presence of Monolaurin
Shoshi Inooka*
Japan Association of Science Specialists, Japan
*Corresponding author: Shoshi Inooka, Japan Association of Science Specialists, Japan
Submission: June 06, 2022;Published: June 14, 2022
.jpg)
Volume11 Issue2June, 2022
Abstract
Techniques for producing artificial cells (DNA crown cells) in which the outside of the membrane is covered with DNA were established in 2012. Such cells can be readily synthesized using sphingosine (Sph)-DNA-adenosine mixtures (designated synthetic DNA crown cells) and can proliferate within egg whites.
However, how synthetic DNA crown cells proliferate within egg white has yet not been clarified in detail. It was previously demonstrated that synthetic DNA (E. coli) crown cells formed assemblies with salts, which implies that synthetic DNA crown cells were reconstructed. Many such synthetic DNA crown cells were observed within these assemblies where they were retained. In this study, I first attempted to clarify whether assembly formation could be produced using specific inorganic salts (NaCl, KCl, MgCl2, and FeCl3 solution) . Then, the process of modified DNA (E. coli) crown cells within the assembly being released after the addition of monolaurin was investigated.
The findings showed that assemblies of synthetic DNA (E. coli) crown cells were formed using these inorganic salts, and a population of modified synthetic DNA crown cells were observed within such assemblies. However, the modified synthetic DNA crown cells within such assemblies were not released in the presence of monolaurin. Indeed, assemblies which contained cells were transformed to crystallike substances after the addition of monolaurin and the cells disappeared. These findings indicated that assembly of synthetic DNA crown cells were formed with inorganic salts and also spontaneously, and that they were produced following the formation of the assembly. In addition, the assemblies were reconstructed with monolaurin whereafter they changed to crystal-like substances.
Keywords: Keywords: Synthetic DNA crown cells; Assembly; Sphingosine-DNA; Adenosine-monolaurin
Introduction
Approaches for generating artificial cells, which are cells covered with DNA, were first reported in 2012 [1,2]. These artificial cells have been designated DNA crown cells [3].
DNA crown cells can be synthesized using biotechnology and synthetically (synthetic DNA crown cells) [4]. These processes were performed using sphingosine (Sph)-DNA and adenosine-monolaurin (A-M). Synthetic DNA crown cells proliferate within egg white and grow into DNA crown cells. To date, several kinds of synthetic DNA crown cells and DNA crown cells have been prepared [5-8]. However, it is unclear how synthetic DNA crown cells proliferate within egg white.
In a previous report [9]. It was shown that the assembly of synthetic DNA crown cells was formed in the presence of salt, which consists of inorganic ions which are present in egg white. In addition, such assemblies also formed spontaneously without the addition of any salt. Many of the synthetic DNA crown cells that were modified were retained within such assemblies. However, one of the challenges highlighted in that study was whether the assembly formed in response to specific inorganic ions. Another challenge involved why synthetic DNA crown cells could not be released from the assembly. Therefore, this study sought to clarify whether such assemblies were formed in the presence of the inorganic ions in NaCl, KCl, MgCl2 and FeCl3. These substances were added to synthetic DNA crown cells and the mixtures were cultured
The results showed that the assemblies of synthetic DNA (E. coli) crown cells were formed in all of the experiments. Then, monolaurin was added to the assemblies that formed using the specific inorganic salts and spontaneously. It was found that most of the assemblies were transformed to crystal-like substances and no modified synthetic DNA crown cells were observed within the assemblies affected by the inorganic salts.
These findings showed that synthetic DNA (E. coli) crown cells were reconstructed using several inorganic salts, as well as spontaneously, and that assemblies containing modified synthetic DNA (E. coli) crown cells were formed. Moreover, modified synthetic DNA (E. coli) crown cells within the assembly were not released after the addition of monolaurin. Indeed, after the addition of monolaurin, the assembly formed crystal-like substances. Thus, synthetic DNA (E. coli) crown cells were reconstructed in the form of assemblies and they were again reconstructed in the form of crystal-like substances. It is suggested that this duality in crown cell formation may be associated with the production of new cells or regeneration of synthetic DNA (E. coli) crown cells
Materials and Methods
Materials
The following materials were used in this study. Sph (Sigma, USA and Tokyo Kasei, Japan), DNA (E. coli, B1 strain; Sigma-Aldrich, USA), adenosine (Sigma, USA and Wako, Japan), monolaurin (Tokyo Kasei, Japan), A-M compound (a compound synthesized from a mixture of adenosine and monolaurin) [10], and NaCl, KCl, MgCl2 and FeCl3 (Hayashi Junyaku, Co, Ltd, Japan).
NaCl, KCl, MgCl2 and FeCl3 were prepared in 20% solutions (w/w) using distilled water, and a 0.1 M solution of monolaurin was prepared using distilled water.
Methods
Preparation of DNA crown cells: The generation of artificial cells using Sph-DNA-A-M was performed as described previously [10]. Briefly, 180μL of Sph (10mM) and 90μL of DNA (1.7μg/μL) were combined, and the mixture was heated twice. A-M compound (100μL) was added to the mixture, which was then incubated at 37 ºC for 15 minutes. Next, 30μL of monolaurin was added, and the mixture was incubated at 37 ºC for another 5 minutes. The resulting suspension was used as synthetic DNA (E. coli) crown cells.
Addition of NaCl, KCl, MgCl2 and FeCl3 to synthetic DNA (E. coli) crown cells: A 25μL aliquot of each NaCl, KCl, MgCl2 or FeCl3 solution was combined with 25μL of synthetic DNA (E. coli) crown cells. The mixture was incubated for 3.0, 18 and 36 hours at 37 ºC, at which point a sample was examined under a microscope.To investigate the effect of monolaurin on synthetic DNA (E. coli) crown cell assembly, 0.25μL of synthetic DNA (E. coli) crown cells were incubated with 25μL of NaCl, KCl, MgCl2 or FeCl3 for 18 hours at 37 °C. Then, 0.25μL of monolaurin (0.1M) was added to each sample and incubated at 37 ℃ for 0 (before incubation), 0.5, 1.0. 3.0 and 18 hours. Control cultures were prepared without the addition of the inorganic salts.
In the microscopic observations, a drop of the synthetic DNA (E. coli) crown cells that were cultured with each inorganic salt was placed on a slide glass and covered with a cover glass. The resulting slide then was observed under a light microscope.
Results and Discussion
Microscopic appearance of synthetic DNA (E. coli) crown cells cultured without inorganic salts
The microscopic appearance of synthetic DNA (E. coli) crown cell cultures was reported previously [9]. However, the appearance of synthetic DNA (E. coli) crown cell cultures may vary between experiments because preparing synthetic DNA (E. coli) crown cells with a consistent appearance is difficult. Therefore, it may be necessary to examine the appearance of synthetic DNA (E. coli) crown cells prepared for each experiment after incubation for 3 and 18 hours.
Figure 1 shows the microscopic appearance of synthetic DNA (E. coli) crown cells after incubation for 3 hours. A typical single synthetic DNA (E. coli) crown cell is shown in Figure 1 (arrows b and d). The cells measured approximately 2-5μm. Larger synthetic DNA (E. coli) crown cells (approximately 10-20μm) were also observed (Figure 1, arrow a). A bent structure was observed in the cell (Figure 1, arrow c).
Figure 1: Microscopic appearance of synthetic DNA (E. coli) crown cells after the incubation of synthetic DNA (E. coli) crown cells for 3 hours. A typical single synthetic DNA (E. coli) crown cell is shown (arrows b and d). The cells measure approximately 2-5μm. Larger synthetic DNA (E. coli) crown cells (approximately 1-20μm) were observed (arrow a). Structure enclosed in the cell with bent shape was observed (arrow c).
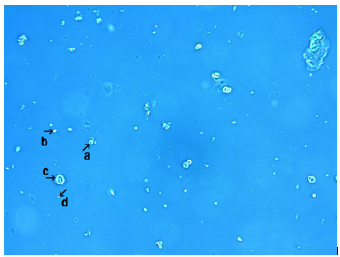
Figure 2 shows the microscopic appearance of synthetic DNA (E. coli) cells after 18h of culture. Crystal-like assemblies (arrow a), and assemblies containing many round or rod-like structures were also observed (arrow b). Ring-like structures (arrow c) and incomplete ring-like structures were observed (arrow d and arrow). A single synthetic DNA (E. coli) crown cell was observed in Figure 2 (arrow e; approximately 10μm).
Figure 2: Microscopic appearance of synthetic DNA (E. coli) crown cells after 18 hours of culture. A crystal-like assembly was observed (arrow a). An assembly containing many round or rod-like structures like was also observed (arrow b). Ring-like structures were observed (arrow c) and incomplete ring-like substances were also observed (arrow d and arrow). A single synthetic DNA (E. coli) crown cell was observed (arrow e; approximately 10μm in size).
Microscopic appearance of synthetic DNA (E. coli) crown cells incubated in the presence of NaCl for 3.0 and 18h at 37 °C
Synthetic DNA (E. coli) crown cells were cultured in the presence of NaCl and observed. Figure 3 showed microscopic appearance of synthetic DNA (E. coli) crown cells after incubation with NaCl for 3 hours.
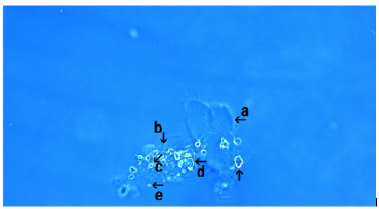
Figure 3: Microscopic appearance of synthetic DNA (E. coli) crown cells after culturing for 3 hours with NaCl. Several crystal-like assemblies were observed (arrow a). Also, many round structures (varying in size and shape) were observed around the crystal-like assemblies (arrow b). Free synthetic DNA (E. coli) crown cells were observed (arrows c and d). The sizes of these cells is approximately 2-5μm.
Several kinds of crystal-like assemblies were observed (arrow a). Also, many round structures (varying in size and shape) were observed around the crystal-like assemblies (arrow b). These round structures were synthetic DNA (E. coli) crown cells, as described in a previous report [9]. Free synthetic DNA (E. coli) crown cells measuring approximately 2-5μm were also observed (Figure 3, arrows c and d).
Figure 4 shows microscopic appearance of synthetic DNA (E. coli) crown cells at 18h after NaCl addition. An assembly (arrow a) consisting of many modified synthetic DNA (E. coli) crown cells (arrows b, g, h and i) or a block of these cells (arrows c, d, e and f) was observed. Cells (arrow i) measured approximately 2-3μm. The figure shows various structures that are produced during the synthesis of synthetic DNA (E. coli) crown cells. As reported previously [9-11], the possible formation of synthetic DNA (E. coli) crown cells is as follows (Figure 4).
Figure 4: Microscopic appearance of synthetic DNA (E. coli) crown cells after culturing with NaCl for 18 hours. An assembly (arrow a) consisting of numerous modified synthetic DNA (E. coli) crown cells (arrows b, h, I and g ) or blocks of these cells (arrow c, d, e and f) were observed. Cell (arrow i) was approximately 2-3μm in size.
Step 1: Sph-DNA fibers are formed as Sph and DNA bind
together.
Step 2: The bunch of Sph-DNA fibers are formed with A-M
compounds.
Step 3: The ring (cells) of the bunch are formed.
All of the structures that were observed in Figure 4 (arrows c, d, e and f) were constituted of Sph-DNA fibers. The structure in Figure 4 (arrows c and d) are like rod-like, indicating the bunch of Sph- DNA fibers.
On the other hand, structures in Figure 4 (arrows e and f) were rod-like and bent, indicating the condition of the bunch before the formation of ring (cells). The structures shown in Figure 4 (arrows b, g, h and i) were round, indicating that cells were formed. Thus, the process of synthetic DNA (E. coli) crown cell formation was clarified by microscopic observation.
Microscopic appearance of synthetic DNA (E. coli) crown cells incubated in the presence of CaCl2
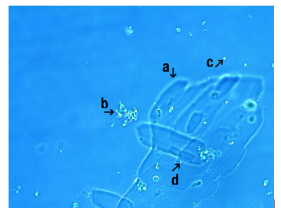
Figure 5 shows the microscopic appearance of synthetic DNA (E. coli) crown cells incubated in the presence of CaCl2 for 18 hours A large assembly (Figure 5, arrow a), small assembly (Figure 5, arrow c), and a crystal-like structure (Figure 5, arrow b) were observed. A free synthetic DNA (E. coli) crown cell measuring 2-3μm was observed (arrow d). The assembly (Figure 5, arrow c) consisted of synthetic DNA (E. coli) crown cells, as indicated in Figure 5 (arrow d). The assembly consisted of numerous modified synthetic DNA (E. coli) crown cells.
Figure 5: Microscopic appearance of synthetic DNA (E. coli) crown cells after culturing with CaCl2 for 18 hours. A large assembly (arrow a) with small assembly (arrow c) and a crystal-like structure (arrow b) were observed. A free synthetic DNA (E. coli) crown cell measuring 2-3μm in size was observed (arrow d).
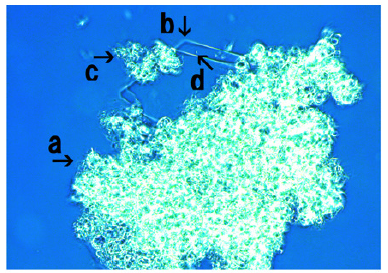
Microscopic appearance of synthetic DNA (E. coli) crown cells incubated in the presence of MgCl2
Figure 6: Microscopic appearance of synthetic DNA (E. coli) crown cells after culturing with with MgCl2 for 18 hours. A large assembly was observed (arrow a). The assembly consists of many modified synthetic DNA (E. coli) crown cells (arrow b) which have different sizes. The size of the cell (arrow c) was approximately 2-3μm.
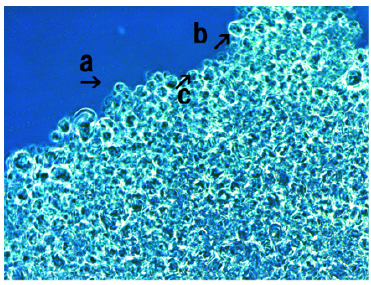
Figure 6 shows synthetic DNA (E. coli) crown cells after incubation with MgCl2 for 18 hours. A large assembly was observed (Figure 6, arrow a). The assembly consisted of numerous modified synthetic DNA (E. coli) crown cells (Figure 6, arrow b) of different sizes. The size of the cell shown in Figure 6 (arrow c) was approximately 2-3μm.
Microscopic appearance of synthetic DNA (E. coli) crown cells incubated in the presence of FeCl3
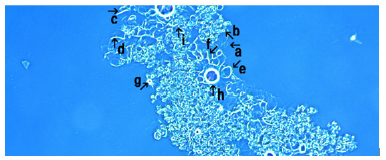
Figure 7 shows synthetic DNA (E. coli) crown cells after incubation with FeCl3 for 18 hours. An assembly (Figure 7, arrow a), cluster of modified synthetic DNA (E. coli) crown cells (Figure 7, arrows b and c) and single modified synthetic DNA (E. coli) crown cell (Figure 7, arrows h and g) were observed. The size of the cell in Figure 7 (arrow h) was approximately 10-12μm. Also, structures in the process of cell formation were observed (Figure 7, arrows d, f and e). It was unclear whether the structures in Figure 7 (arrow i) were associated with the formation of cells.
Figure 7: Microscopic appearance of synthetic DNA (E. coli) crown cells after culturing with FeCl3 for 18 hours. An assembly (arrow a), a cluster of modified synthetic DNA (E. coli) crown cells (arrows b and c) and a single modified synthetic DNA (E. coli) crown cell (arrows h and g) were observed. The size of the cell (arrow h) was approximately 10-12μm. Also, structures associated with cell formation were observed (arrows d, e and f).
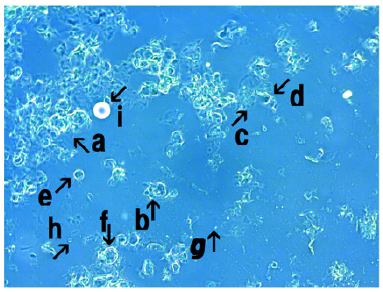
Microscopic appearance of synthetic DNA (E. coli) crown cells in a mix-culture of spontaneously assembled synthetic DNA (E. coli) crown cells with monolaurin
Monolaurin was added to synthetic DNA (E. coli) crown cells which were incubated for 18 hours. Then, the mixtures were incubated for 0 (before incubation), 1.0 and 3.0h at 37 ℃.
Figure 8 shows the microscopic appearance of synthetic DNA (E. coli) crown cells after 0h of monolaurin addition. Crystal-like substances were observed in most of Figure 8 (Figure 8, arrow a), suggesting that most of cultured synthetic DNA (E. coli) crown cells were transformed into a crystal-like substance. On the other hand, the fragments of the substances which were accumulated were observed (Figure 8, arrow b). Structures which may be not transformed to crystal-like substances were observed in Figure 8 (arrows c and d). Arrow c in Figure 8 may consist of small round structures which were observed in Figure 8 (arrow d). Structures which contained several particles were observed in Figure 8 (arrow e). However, it is unclear whether it occurs due to the addition of monolaurin. A single synthetic DNA (E. coli) crown cell was observed in Figure 8 (arrow f) (approximately 10-20μm). Here, it was showed that the transformation immediately occurred before incubation. Therefore, the change to crystal like substances may occur without incubation
Figure 8: Microscopic appearance of synthetic DNA (E. coli) crown cells in a mixed culture of spontaneously assembled synthetic DNA (E. coli) crown cells with monolaurin. Monolaurin was added to synthetic DNA (E. coli) crown cells, which were incubated for 18 hours. Then, the mixtures were incubated for 0 hour at 37 0C (before incubation). Crystal-like substances were observed in most of Figure 8 (arrow a). Fragments of the substances which were accumulated were observed (arrow b). Structures which may be not transformed to crystal like substances were observed (arrows c and d). Structures containing several particles are shown by arrow e. A single synthetic DNA (E. coli) crown cell is shown by arrow f (approximately 10-20μm).
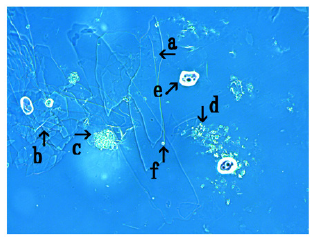
Figure 9: Microscopic appearance of synthetic DNA (E. coli) crown cells at 1.0 hour after monolaurin was added to synthetic DNA (E. coli) crown cells, which were cultivated for 18 hours at 37 0C. Large crystal-like substances were observed (arrow a). Structures which may be not transformed were observed (arrows b and c). A single synthetic DNA (E. coli) crown cell (2-3μm size) was observed (arrow d).
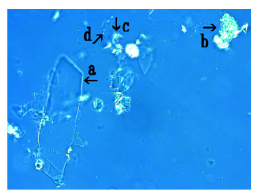
Figure 9 shows the microscopic appearance of synthetic DNA (E. coli) crown cells after 1.0h of monolaurin addition. A large crystal-like substance was observed (Figure 9, arrow a). Structures which may be not transformed were also observed (Figure 9 arrows b and c). A structure which was observed in Figure 9 (arrows b and c) may be related to the assembly of synthetic DNA (E. coli) crown cells. A single synthetic DNA (E. coli) crown cell (approximately, 2-3μm) was observed (Figure 9, arrow d).
Figure 10 showed the microscopic appearance of synthetic DNA (E. coli) crown cells after 3.0h of monolaurin addition. Figure 10 (arrow a) shows that many crystal-like substances be accumulated.
Also, structures which were not transformed were observed on the crystal-like substances (Figure 10, arrow b) and might consist of synthetic DNA (E. coli) crown cells. A single synthetic DNA (E. coli) crown cell (approximately 2-3μm) was observed (Figure 10, arrow c). Thus, most of the assemblies of synthetic DNA (E. coli) crown cells which were spontaneously formed were transformed into crystal-like substances, and a parts of these were not transformed and retained the shape of synthetic DNA (E. coli) crown cells.
Figure 10: Microscopic appearance of synthetic DNA (E. coli) crown cells at 3 hours after monolaurin addition to synthetic DNA (E. coli) crown cells, which were then cultivated for 18 hours at 37 0C Many crystal-like substances were accumulated (arrow a). Structures which were not transformed were observed on the crystal-like substances (arrow b) and may consist of synthetic DNA (E. coli) crown cells. A single synthetic DNA (E. coli) crown cell (approximate size is 2-3μm) is shown (arrow c).
Microscopic appearance of synthetic DNA (E. coli) crown cells in mixed cultures of cells cultivated for 36h with monolaurin
Next, to study the effect of culture period on the formation of crystal-like substances after incubation with monolaurin, synthetic DNA (E. coli) crown cells were incubated for 36h at 37 ℃ and monolaurin was then added and incubated for 0.5h at 37 ℃.
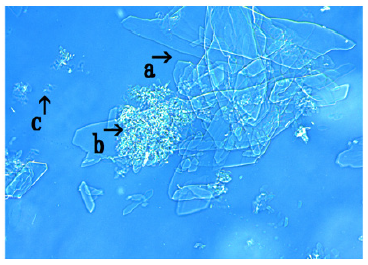
The assembly of crystal-like substances was observed (Figure 11, arrow a). The fragments of crystal-like substances of various shapes and sizes was observed within the assembly (Figure 11, arrow b). A clear structure (arrow d) and rod-like substances were observed (Figure 11, arrow c). However, it is unclear whether this rod-like substance was a crystal-like substance. A single DNA (E. coli) crown cell (approximately 15-20μm) was observed (arrow e).
Figure 11:Microscopic appearance of synthetic DNA (E. coli) crown cells at 0.5 hours after the mixtures of synthetic DNA (E. coli) crown cells cultivated for 36 hours with monolaurin. An assembly of crystal-like substances was observed (arrow a). The fragments of crystal-like substances in various shape and sizes was observed within the assembly (arrow b). The clear structures was observed (arrow d). Structure like stick was observed (arrow c). A single DNA (E. coli) crown cell (Approximate size 15- 20μm) was observed (arrow e).
The findings suggested that crystal-like substances of a new type occurred after synthetic DNA (E. coli) crown cells were incubated for 36h and treated with monolaurin.
.Microscopic examination of mix-cultures of NaClassociated assemblies of synthetic DNA (E. coli) crown cells in the presence of monolaurin
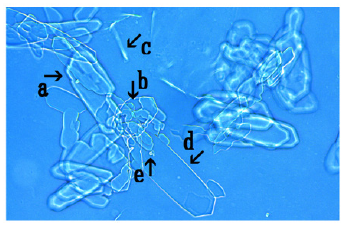
Figure 12:Microscopic appearance of the mix-cultures of the NaCl-associated the assembly of synthetic DNA (E. coli) crown cells at 3 hours after monolaurin addition. Structures consisting of a crystal-like substance and a non-transformed substance were observed (arrow a). Synthetic DNA (E. coli) crown cells were observed within such structures (arrows b and d). Crystal-like structures were observed (arrow c). A single synthetic DNA (E. coli) crown cell (approximately 10- 20μm) was observed (arrow e).
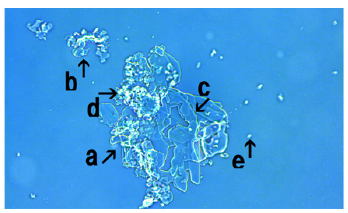
Figure 12 shows the microscopic appearance of NaCl treated synthetic DNA crown cells at 3.0h after monolaurin addition. A mixture of crystal-like substances and non-transformed substances were observed (Figure 12, arrow a). Synthetic DNA (E. coli) crown cells were observed within such structures (Figure 12, arrows b and d). Crystal-like substances (arrow c) and a single synthetic DNA (E. coli) crown cell (approximately 10-20μm) were observed (Figure 12, arrow e).
Microscopic appearance of mixed cultures of CaCl2- associated assemblies of synthetic DNA (E. coli) crown cells in the presence of monolaurin
Figure 13 shows the appearance of synthetic DNA (E. coli) crown cells at 18h after monolaurin addition. Crystal-like substances (arrow a), and structures which were not transformed to crystallike substances and might consist of synthetic DNA crown cells were observed (Figure 13, arrow b). A single synthetic DNA (E. coli) crown cell (approximately 10-20μm) was also observed (arrow c).
Figure 13:Microscopic appearance of the mix-cultures of the CaCl2-associated assembly of synthetic DNA (E. coli) crown cells at 18 hours after monolaurin addition. A crystal-like substance was observed (arrow a). Structures which were not transformed to crystal-like substances and potentially consisting of synthetic DNA crown cells were observed (arrow b). A single synthetic DNA (E. coli) crown cell (approximately 10-20μm) was observed (arrow c).
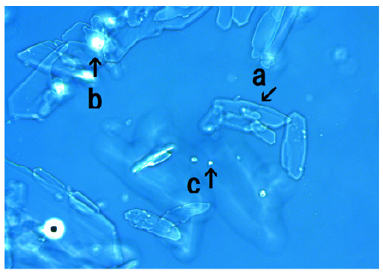
Microscopic appearance of mixed cultures of MgCl2- associated assemblies of synthetic DNA crown cells in the presence of monolaurin
Figure 14 shows microscopic appearance of synthetic DNA crown cells at 18h after monolaurin addition. Crystal-like substances (arrow a), and structures which were not transformed to crystal-like substances and might consist of synthetic DNA crown cells were observed (arrow b). A single synthetic DNA (E. coli) crown cell (approximately 10-20μm) was observed (arrow c).
Figure 14:Microscopic appearance in the mix-cultures of the MgCl2-associated assembly of synthetic DNA crown cells at 18 hours after monolaurin addition. A crystal-like substance was observed (arrow a). A structure that was not transformed and which might be a cluster of synthetic DNA (E. coli) crown cells was observed (arrow b). A single synthetic DNA (E. coli) crown cell (approximately, 10~20μm) was observed (arrow c).
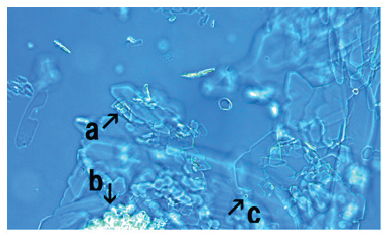
Microscopic appearance of mixed cultures of FeCl3- associated assemblies of synthetic DNA crown cells in the presence monolaurin
Figure 15:Microscopic appearance of the mix-cultures of the FeCl3-associated assembly of synthetic DNA crown cells at 18 hours after monolaurin addition. A crystal-like substance was observed (arrow a). A large structure that was not transformed to a crystal-like substance was observed (arrow b). Several synthetic DNA (E. coli) crown cells were observed within such structures (arrow c). A single synthetic DNA crown cell was observed (approximately 10-20μm) (arrow d).
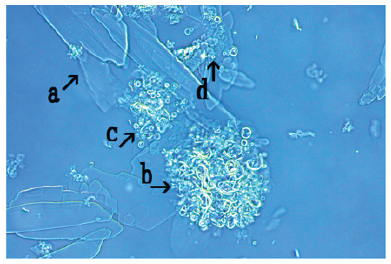
Figure 15 shows the appearance of synthetic DNA (E. coli) crown cells at 18h after monolaurin addition. A crystal-like substance (arrow a) and a large structure that was not transformed to a crystal-like substance was observed (Figure 15, arrow b). Several synthetic DNA (E. coli) crown cells were observed within the structures (Figure 15, arrow c). A single synthetic DNA crown cell was observed (approximately 10-20μm) (arrow d). In the present experiments, it was examined whether synthetic DNA (E. coli) crown cells formed assemblies in the presence of the inorganic salts NaCl, CaCl2, MgCl2 and FeCl3.
In a previous report [9], it was shown that assembly formation was observed in mixtures of synthetic DNA (E. coli) crown cells with salts, and it was tested whether the ions contained in salt have the same effects on assembly formation. Here, the effect of salts was confirmed to have the same effects (i.e., assembly formation was observed). It may be not difference between salt and specific materials on the formation of the assembly. However, the assembly which contained many synthetic DNA crown cells may be formed in the presence of salts, especially NaCl, CaCl2 and MgCl2. The synthetic DNA crown cells within such assemblies may differ from the original synthetic DNA crown cells, as described previously [9], and these crown cells are referred to here as modified synthetic DNA crown cells.
Most modified synthetic DNA (E. coli) crown cells are retained within the assembly. If these cells are released from the assembly, then they could be regarded as regenerated cells or newly formed cells (possibly DNA (E. coli) crown cells). Therefore, in the second experiment, it was examined whether these retained cells were released from the assembly. Although enzymes can be used for cell separation, monolaurin was used in this study. Monolaurin was used to prepare synthetic DNA (E. coli) crown cells and many free cells were observed in assemblies which were formed in the use of such materials [12,13].
When monolaurin was added to synthetic DNA (E. coli) crown cells, both in the presence or absence of salts, crystal-like substances were observed in immediately after the addition. In 18 hours of addition, most of the assemblies changed to clear crystallike substances.
However, the formation of crystal-like substances may not be uniquely attributed to monolaurin. As shown in Figure 3, crystallike substances were formed when NaCl was added to synthetic DNA (E. coli) crown cells before the addition of monolaurin. However, it was clear that monolaurin was effective for inducing the formation of crystal-like substances, because most of the assemblies changed to crystal-like substances with the addition of monolaurin. Though it was expected that such cells would be released from the assembly by adding monolaurin, they remained within the assembly and disappeared with the formation of crystal-like substances. At present, it is unclear how the formation of such substances was associated with regenerated cells.
However, it is clear that synthetic DNA (E. coli) crown cells were reconstructed and formed the assembly. Moreover, such reconstructed synthetic DNA (E. coli) cells were again constructed with monolaurin and formed crystal-like substances. In this way, synthetic DNA (E. coli) crown cells were formed twice. First, such cells were produced in the formation of the assembly, and second, such cells were again produced during the formation of crystallike substances. The identity of the crystal-like substances that were observed in these experiments is unknown. However, it was demonstrated previously [3,4] that crystal-like substances were formed in the presence of Sph-DNA. Therefore, it is possible that these substances consist of Sph-DNA. Future research will examine whether new cells (like DNA crown cells) can develop from synthetic DNA (E. coli) crown cells that are constructed twice.
References
- Inooka S (2012) Preparation and cultivation of artificial cells. App Cell Biol 25: 13-18.
- Inooka S (2016) Preparation of artificial cells using eggs with sphingosine-DNA. J Chem Eng Process Technol l7: 277.
- Inooka S (2016) Aggregation of sphingosine-DNA and cell construction using components from egg white. Integrative Molecular Medicine 3(6): 1-5.
- Inooka S (2017) Biotechnical and systematic preparation of artificial cells. The Global Journal of Researches in Engineering 17(1).
- Inooka S (2017) Systematic preparation of bovine meat DNA crown cells. App Cell Biol Japan 30: 13-16.
- Inooka S (2018) Systematic preparation of DNA (Akoya pearl oyster) crown cells. App Cell Biol Japan 31: 21-34.
- Inooka S (2019) Preparation of DNA (Nannochloropsis species) crown cells using eggs, sphingoshin and DNA, and subsequent cell recovery. App Cell Biol Japan 32: 55-64.
- Inooka S (2014) Preparation of artificial human placental cells. App Cell Biol 27: 43-49.
- Inooka S (2022) Assemblies formation of synthetic DNA (E.coli) crown cells with Salt. “Reconstruction and regeneration of synthetic DNA crown cells”. American Journal of Biomedical Science & Research 16(1): 111-120.
- Inooka S (2017) Systematic preparation of artificial cells (DNA Crown Cells). J Chem Eng Process Technol 8(1): 1-4.
- Inooka S (2018) Theory of cyto-organisms generation-Artificial cells produced with egg. The discover and synthesis of DNA crown cells (In Japanese), Daigaku kyouiku Press, Okayama, Japan.
- Inooka S (2021) Assembly and bacterial growth suppression in the Co-cultures of synthetic DNA (Akoya Pearl Oyster) crown cells with Bacillus subtilis. Applied Cell Biology Japan 4: 67-84.
- Inooka S (2022) Assemblies in synthetic DNA (Nannochloropsis Species) crown cells with Bacillus subtilis. Acta Scientific Microbiology 5(1): 1-8.
© 2022 Shoshi Inooka. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






