- Submissions

Full Text
Novel Research in Sciences
Recent Trends in Fullerenes Biomedical Applications
Nicholas Mulqueen1, Kevin Sneed2 and Yashwant Pathak2,3*
1Department of Medical Engineering, USA
2Taneja College of Pharmacy, USA
3Faculty of Pharmacy, Indonesia
*Corresponding author: Yashwant Pathak, Taneja College of Pharmacy, USA
Submission: March 03, 2022;Published: April 14, 2022
.jpg)
Volume10 Issue5April, 2022
Abstract
Fullerenes have enjoyed almost four decades of research and development since they were discovered in 1985. C60 the first characterized fullerene is considered pristine in its state. Fullerenes, as the third allotrope of carbon come in many sizes, have shown exceptional development over time in biomedical applications utilizing their outstanding physical, chemical, and antioxidant effects. However, fullerenes are highly hydrophobic making them difficult to utilize in biomedical sciences. With the addition of functional groups, water-soluble derivatives have been created but not without the potential to have toxic effects on organic systems. This review covers recent trends in biomedical applications of fullerenes, their derivatives, while not a complete overview of fullerenes as the field contains an exhaustive amount of material concerning them, this will be a brief glimpse into them to give the reader a little insight into the impressive prospects that fullerenes have to offer.
Keywords: Keywords: Fullerenes; Biomedical applications; Synthesis of fullerenes; Properties of fullerene useful for biomedical applications; Recent trends in fullerene researches
The Introduction
Buckminsterfullerene, buckyballs, or C60 fullerene was first discovered in 1985, in an attempt to make long-chain carbon molecules in hopes to understand how they would form under interstellar conditions [1]. The name Buckminsterfullerene pays homage to the American architect Buckminster Fuller, who was famous for his designs of geodesic domes [2]. Fullerene is known as the third form of carbon after graphite and diamond [3]. The shape can be described as that of a soccer ball, a truncated icosahedral with 12 pentagon faces and 20 hexagon faces, where each vertex of the shape is an sp2 hybridized carbon atom. Buckminsterfullerene, now known colloquially as Pristine C60, is not the only fullerene to exist, with hundreds of stable configurations found in the family of fullerenes. It’s been found that fullerenes range in sizes from C20 to C2160 in configurations that are of the formula 20+2n carbons where there are n number of hexagon faces in the structure with symmetrical fullerenes always having 12 pentagon faces [4,5]. While this seems like a large range of structures, for biomedical applications the research and development of fullerenes have mainly been in the range of C60 to C100. This is due largely to the nature of the production of fullerenes with C60 having the largest abundance under synthesis conditions with other fullerenes (i.e., C70, C76, C80…) being produced in significantly smaller quantities [3].
C60 the first fullerene
The size of pristine C60 is an average of 0.71nm of external diameter. It is considered a 0-dimensional carbon structure being the smallest of all carbon nanostructures known. The spherical shape of pristine C60 is maintained by the bond angle between the carbon angles. While sp2 hybridized, the closed cage structure changes the bond angle to the degree that it is actually in configuration between sp2 and sp3 hybridization [6]. Furthermore, the bond lengths between the pentagon faces and hexagon faces of the fullerene differ. For hexagon adjacent faces the bond length is 1.38Å and 1.45Å between the pentagon to hexagon connecting carbons termed 66 bonds and 56 bonds respectively [6].
Synthesis of the fullerene
The first method to synthesize fullerenes in bulk form was developed in 1990 by Krätschmer et al. [7]. Since then, few other methods have been used to create fullerenes. Among these general methodologies are vaporization by discharge (arc or plasma), laser ablation, naphthalene pyrolysis, carbon vapor deposition, and combustion with organic carbon [8-13]. All these processes aim to manipulate graphite in ways that fragment it into forming fullerene spheres. In the method described by Krätschmer et al. [7], graphite rods are used to produce graphitic soot inside of a pressurized vessel using a helium atmosphere at 100Torr. The soot is collected and mixed with benzene which allows for the separation of fullerenes by dissolution. The mixture is heated gently to dry and the result is a dark-colored crystalline material. It is noted in the work that 100mg of material can be produced in a day by this method and the resulting ratio of C70 to C60 can be anywhere from 0.1 to 0.02.
Similar to the Krätschmer method described above, arc and plasma discharge involves the use of creating graphitic soot using graphite rods. These processes are similar in that they use a cooled collection chamber pressurized with helium and a DC arc welder to generate the current. Their differences lie in that arc discharge occurs in pulsed increments to allow cooling and plasma discharge is continuous except when the negative electrode needs to be cleaned due to slag build-up. All these methods require organic solvents to extract and separate the fullerenes by size [8,9]. Laser ablation has a similar schematic of setup where graphite is vaporized with a laser inside of a collection chamber with an inert gas flowing through it (e.g., Argon). The vaporized graphite collects on a cooled copper surface inside the vessel and then can subsequently be collected and the fullerenes separated from other carbon by-products [10].
Naphthalene pyrolysis and carbon vapor deposition method
Naphthalene pyrolysis and carbon vapor deposition share a similar mechanism for the synthesis of fullerenes. Both methods use a heated chamber and an ignition point to pyrolyze an organic carbon. With naphthalene pyrolysis, a 40cm long silica tube is used as the chamber. The solid naphthalene is heated gently with a butane torch on one end and the gas is allowed to pass over the ignition point (the other end heated ~1000 ˚C with another torch) and into a cooled collection vessel. The product is collected, and the process is repeated until the product formed is a black solid [11,12]. In the carbon vapor deposition method, a chamber is designed with an ignition point (a tungsten filament ~2000 ˚C) which is located adjacent to a substrate point (steel, 900-1000 ˚C) and pressurized methane gas and hydrogen are passed through. The fullerene film forms on the substrate and is collected for separation. Lastly, the combustion method is said to achieve high yields of fullerene suitable for commercial production [6]. This method was first implemented by Howard et al. [13] in which it was demonstrated that a sooting flame could produce a sizeable quantity of fullerene products with a relatively low amount of fuel. Howard et al. note in their findings that what sets this method apart from arc vaporization is that control of the C70/C60 ratio can be controlled through control of the flame conditions.
Properties of fullerenes
One of the many keys to the use of fullerenes in biomedical applications is the properties they display. Pristine fullerenes show several useful physical and electrical properties that make them suitable nanomaterial for biomedical applications. Going back to the structure of the fullerene, pristine fullerene is the smallest electronically stable fullerene by the Isolated Pentagon Rule (IPR). This rule denotes that when all pentagons are isolated from each other in the fullerene structure then the resulting fullerene will be electronically stable, destabilization caused by the resonance of pibonds occurs when pentagons are adjacent to each other [14]. As mentioned previously, the hybridization state being between sp2 and sp3 leads to the electronegative nature of pristine fullerenes as the pi-orbitals extend farther out than inside the molecule [6,14]. These details of this structure affect fullerene’s electrical and chemical properties.
Electrochemical properties of fullerene
One of the pristine fullerene’s most notable electrochemical properties is how it interacts with radicals. In the presence of UV light, pristine fullerene exhibits pro-oxidant activity with the ability to create Radical Oxygen Species (ROS). Counter to this, fullerenes under the right conditions display powerful antioxidant properties being dubbed a “radical scavenger” or “radical sponge”. The ability to do so is based on a large amount of double-bonded carbon and the fullerene resonance forms, being able to accept between 1 and 34 free radical groups [6,15]. This powerful antioxidant mechanism makes the fullerene desirable even in the face of its pro-oxidant mechanism.
Hydrophobic nature of fullerene
Another property of fullerene to contend with in making a suitable biomedical nanomaterial is its hydrophobic nature. Pristine fullerene is not water-soluble so use as a biomedical device is difficult. Because of their hydrophobicity and reactivity, fullerenes tend to form aggregates when exposed to polar solvents, such as those found in aqueous solutions or within living systems. This tends to make them more of a burden and tends to present with levels of toxicity [2,5]. Luckily with the number of double-bonded carbons in the fullerene structure, they can react well with many functional groups allowing for the creation of derivatives. Through functionalization, the fullerene can retain its unique properties but also has enhanced water solubility through the addition of hydrophilic functional groups [16].
Functionalization
Functionalization of fullerenes is a key to unlocking their use for all biomedical applications. The goals of functionalization for fullerenes are to stabilize it under ambient conditions, control its hydrophobicity and water solubility, and select for desired properties [17]. Functionalization of fullerenes can be accomplished by exohedral, endohedral, or a combination of both methods [2]. Exohedral functionalization involves the addition of functional groups to the outside fullerene structure. There are four general descriptions for the addition to the fullerene that take place. The first is the addition of a group by covalent bonding to a single carbon on the fullerene. Next is the addition of a functional group by epoxy bridging utilizing [2+1] cycloaddition linking two fullerene carbons to a single atom of a functional group with a maximum number of these additions being 18. The third is [2+n] cycloaddition where n is greater than or equal to 2. Lastly, the substitution of atoms within a fullerene structure can be done to the fullerene structure [17].
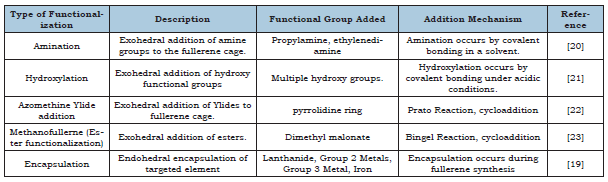
Endohedral functionalization involves encapsulating an atom within the fullerene cage usually during the synthesis of the fullerene. It was discovered shortly after the initial discovery of pristine fullerenes during a trial to vaporize graphite that had been impregnated with lanthanum [18]. Now it is known that many different elements can be encapsulated within a fullerene to usually create metallofullerenes. These elements are in the lanthanide series (La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu) as well as group 2 metals (Ca, Sr, Ba), group 3 metals (Sc, Y, La), Ti, and Fe [19]. Encapsulation is usually done under the same conditions as the creation of pristine fullerene occurring under arc discharge or laser ablation with doped graphite to produce a mixture of empty fullerenes and metallofullerenes [19]. Table 1 is a brief overview of the simple functionalization strategies outlined above. There is an exhaustive list of derivative products that have been developed using fullerene as the core so consider these listed to be that of either the simplest or some of the first functionalized fullerenes to be discovered.
Table 1: Brief overview of the simple functionalization of fullerenes.
Toxicity
The hydrophobic nature, as well as radical-producing properties, lend to the prediction of the potential toxicity of fullerenes. The topic of fullerenes toxicity had long been debated almost since the time of its discovery. Early on it can be shown that fullerenes may not pose an inherent risk in studies done on mice. A 1993 study showed that there was no cause for concern when topically applied fullerenes were found to cause no DNA damage and cause no tumor formation in the skin of mice [20-24]. A study following would also test the uptake of human keratinocytes for the accumulation of fullerenes in an organic solvent. The results concluded that there was no acute toxicity noted in human keratinocytes and fibroblasts [25].
More research would prove to contradict these early findings. As shown previously functionalization is necessary to make fullerenes acceptable for use in biomedical applications. Functionalization can also play a role in increasing or decreasing cytotoxic effects. A study from the early 2000s revealed that the degree of functionalization decreases with the increase in functional groups allocated to the fullerene [26]. A mechanism by which the water- soluble fullerenes were causing toxicity was found a year later. The ability to create ROS in vitro caused lipid peroxidation to occur leading to the cytotoxicity of dermal fibroblast, liver cancer cells, and neuronal astrocytes. The peroxidation caused damage to the integrity of the cell membrane leading to cell death. The same study also showed that the use of an antioxidant such as L- ascorbic acid leads to the elimination of peroxidation that occurs in the cell membrane [27]. Additionally, when compared to other nano-carbon structures (single-walled and multi-walled carbon nanotubes) fullerenes were found to be the least cytotoxic [28]. In relation to potential effects on the environment, the effects of fullerenes and nanomaterials are widely unknown Studies showing the effects of fullerene on microbiota are important as low-level organisms affected by nanomaterials could cause ecological shifts in biomes. In evaluating effects on microbial life. Lyon et al. demonstrated the bactericidal effects of pristine fullerene [29]. This study was meant to not only test the potential effects of fullerene nanoparticles on microbial life but to stress the importance of handling to prevent unintentional exposure to the environment.
As mentioned before, much debate over the actual cytotoxic effects of fullerenes are still being explored. One such study shows that in rats with toxicity induced by CCL4, pristine fullerene solubilized into olive oil doubled their life span compared to olive oil and the control group [30,31]. Nevertheless, many studies show that there exist toxic and antioxidant effects of fullerenes so it is application dependent on how fullerenes and their derivatives can be used in biomedical sciences.
Applications
Nanomaterials are a vastly growing industry as of late with billions of dollars going toward research and development. Fullerenes can be found in many industries besides biomedical interests, being explored for applications such as wastewater treatment or organic solar cell construction [32,33]. The role that fullerenes plays in the biomedical world is of utmost importance though, as it can be used in many applications effectively. Among these include highly effective antioxidation, cancer therapy, gene and drug delivery, antiviral and antibacterial activity, and imaging [2,15]. To start the ROS scavenging ability of fullerenes plays an important role as a highly effective antioxidant being able to be utilized in many therapies. ROS species and oxidation have been implicated as causative agents in many conditions including cancer, autoimmunity, atherosclerosis, diabetes, and neurodegenerative disorders [15]. An early study suggested ROS scavenging by fullerene derivatives as a way to mitigate the oxidation and apoptosis of cortical neurons. Dugan et al. provided evidence of radical scavenging that led to neuroprotective activity in cultured cortical neurons using fullerenol derivatives [34].
Another example, in cancer, fullerene molecules can modulate the tumor microenvironment and inhibit the proliferation of cancer cells. Cancer cells are prone to creation in the highly oxidative environment in which they are so the ROS scavenging ability of fullerene has been remarkable in terms of treatment. It has also been shown that Gd@C82(OH)22 is specifically useful in this application having highly potent ROS scavenging ability in the highly oxidative tumor microenvironment [35].
Fullerenes as gene and drug delivery vehicle
As a gene and drug delivery vehicle, fullerene can be used to exploit the Enhanced Permeability and Retention (EPR) effect due to its size. Conjugation of drugs can occur on the outer cage of the shell by either direct attachment or by conjugation onto a functionalized group. In one such example, Doxorubicin (DOX) is conjugated to pristine fullerene to yield a DOX: C60 complex. DOX has inherent toxicity at higher doses and it is believed that it is caused by potential ROS activity by DOX. Since fullerene is found to scavenge radicals, it would be appropriate to conjugate these for research. It was found that the DOX fullerene conjugation ultimately led to more DOX located in the tumor and a longer treatment time as the conjugation slowed the release rate of DOX [36,37]. Shi et al. [38]. also demonstrate the ability to create targeted fullerene derivatives. Using a polyethyleneimine fullerene (C60-PEI) the targeting is done by the conjugation of folic acid as folic receptors are overexpressed in malignant tumor cells. Docetaxel is adsorption to the fullerene derivative was also done to increase tumoricidal activity. The results showed that the fullerene derivative with docetaxel had more efficacy than docetaxel or the fullerene derivative separately.
Bactericidal effects of fullerenes
As mentioned previously was the evidence for bactericidal effects of fullerenes. Fullerene has also displayed promising actions against viral targets. One such example is against HIV and hepatitis C virus (HCV) [39]. In the study, Mashino et al. [39] used cationic, anionic, and amine-derived fullerenes to test the antiviral effects of fullerene on HIV and HCV. It was found that fullerene was effective and inhibiting HIV reverse transcriptase and HCV RNA polymerase activity. Metallofullerenes have shown to work well in conditions needing imaging. Bolskar et al. [40] demonstrate the first use of a metallofullerene in their work. The fullerene Gd@C60[C(COOH)2]10 is used as an MRI contrast agent in vivo in a rodent model. The biodistribution could be noted and it was found to be the first fullerene derivative that did not target and localize in the reticuloendothelial system and did not form aggregates within the solution.
Recent trends in fullerene researches
Since the start of the new decade, there is still interest in the
use of fullerenes in biomedical applications. The following list details
five research studies that have been completed on fullerenes
in the last 2 years:
a. Pristine fullerene C60 is used in STZ- diabetic rats to determine
if it can reduce oxidative stress caused by diabetes. It was
found that fullerenes in fact displayed a neuroprotective effect protecting hippocampal cells and mitigating effects of hypoglycemic
stress displayed by reducing oxidative stress, apoptotic effects, and
increased autophagy flux [41].
b. Determining the effects of fullerene on mice with subcortical
ischemic vascular dementia versus that of sham mice. The
findings show that the mice with dementia had improved cognitive
ability in a Morris water maze than that of the sham mice. It is discussed
that this may be due to the amount of oxidative stress in the
brains of mice with dementia versus that of sham mice [42].
c. The effects of olive oil extracted C60 on the gut microbiota
over a 12-week period. The findings showed that gut microbiota
had in fact changed, to improve serum lipid profile lowering triglycerides
and increasing HDL cholesterol but increased oxidative
markers in the brain and in serum as well as increased insulin resistance
[43].
d. Effects of a fullerene derivative of the authors’ design
are tested and found to have antiviral and anti-myogenic effects.
The fullerene derivative they have dubbed “FPA” is noted to have
been tested against 10 different virus lines including those in the
Coronaviridae family showing promising results in the time of the
pandemic. The authors note that the myogenic differentiation of
mesenchymal stem cells is a first for water-soluble fullerenes [44].
e. Effects of C70 hydroxyl functionalized fullerenes tested to
alleviate silicosis caused by the inhalation of crystalline silica particles
using a silicosis mouse model. The findings show that ROS
scavenging helps alleviate inflammation caused by crystalline silica
[45].
Conclusion and Future Prospectsn
Fullerenes in there almost 40 years have shown that it still holds a lot of promise in biomedical sciences as well as a lot of mystery. Fullerenes with their highly unique physical and electrical chemistry can be exploited heavily by functionalization to create a nanoparticle that works in almost every facet of biomedical science applications. The mystery lies in what we still do not know about the fullerene, the way to control its production or functionalization, and how to perfect it to make it the most useable for all applications. It holds greater mystery as to under what conditions it may be toxic and if we may even be able to use it due to environmental concerns. Fullerenes, for all its promise surrounded by great mysteries that are yet to be uncovered, will be untold with time and hopefully, another 40 years of research will help to expose all the mysteries surrounding them.
As for prospects, there lies a great many. The need for a reliably consistent way to produce fullerenes of select sizes might be a great place to start. In 40 years, the instrumentation has become more precise so as far as controlling the processes that are currently utilized, there may be a solution to creating large consistent yields of fullerenes. Toxicity research is also a must. As environmental protection policies become more stringent in the face of ecological destruction, the risk must be evaluated for not just fullerenes but all nanomaterials on the toxic effects they present to the environment. These are two places to start really looking into how to improve upon fullerene research and development so it may be continued well into the future.
References
- Kroto H, Heath J, O Brien, Curl SRF, Smalley RE (1985) C60: Buckminster fullerene. Nature 318: 162-163.
- Goodarzi S, Da Ros T, Conde J, Sefat F, Mozafari M (2017) Fullerene: Biomedical engineers get to revisit an old friend. Materials Today 20(8): 460-480.
- Taylor R, Hare JP, Abdul Sada AK, Kroto HW (1990) Isolation, separation and characterization of the fullerenes C60 and C70: The third form of carbon. Journal of the Chemical Society, Chemical Communications, p. 1423.
- Dunlap BI, Zope RR (2006) Efficient quantum-chemical geometry optimization and the structure of large icosahedral fullerenes. Chemical Physics Letters 422(4-6): 451-454.
- Sergio M, Behzadi H, Otto A, Vander SD (2013) Fullerenes toxicity and electronic properties. Environ Chem Lett 11: 105-118.
- Georgakilas V, Perman JA, Tucek J, Zboril R (2015) Broad family of carbon nanoallotropes: Classification, chemistry, and applications of fullerenes, carbon dots, nanotubes, graphene, nanodiamonds, and combined superstructures. Chemical Reviews 115(11): 4744-4822.
- Krätschmer W, Lamb L, Fostiropoulos, K, Donald R Huffman (1990) Solid C60: A new form of carbon. Nature 347: 354-358.
- Hare JP, Kroto HW, Taylor R (1991) Preparation and UV/visible spectra of fullerenes C60 and C70. Chemical Physics Letters 177(4-5): 394-398.
- Scrivens WA, Tour JM (1992) Synthesis of gram quantities of C60 by plasma discharge in a modified round-bottomed flask. Key parameters for yield optimization and purification. The Journal of Organic Chemistry 57(25): 6932-6936.
- Curl RF, Smalley RE (1988) Probing C60. Science 242(4881): 1017-1022.
- Taylor R, Langley G, Kroto H, David Walton RM (1993) Formation of C60 by pyrolysis of naphthalene. Nature 366: 728-731.
- Kleckley S, Wang H, Oladeji I, Chow L, Daly TK, et al. (1997) Fullerenes and polymers produced by the chemical vapor deposition method. ACS Symposium Series, pp. 51-60.
- Howard J, McKinnon J, Makarovsky Y, Lafleur AL, Johnson ME (1991) Fullerenes C60 and C70 in flames. Nature 352(6331): 139-141.
- Hirsch A (1999) Principles of fullerene reactivity. Topics in Current Chemistry, pp. 1-65.
- Torres VM, Srdjenovic B (2011) Biomedical application of fullerenes. In: Verner CBRF (Ed.), Handbook on Fullerene: Synthesis, Properties and Applications, pp. 199-239.
- Ros TD, Prato M (1999) Medicinal chemistry with fullerenes and fullerene derivatives. Chemical Communications, pp. 663-669.
- Efstratia M, Michela R, Manesh Z, Roberto M (2016) Solid state physicochemical properties and applications of organic and metallo-organic fullerene derivatives. Current Organic Chemistry 20(6).
- Heath JR, Brien O, Zhang SC, Liu Q, Curl Y, et al. (1985) Lanthanum complexes of spheroidal carbon shells. Journal of the American Chemical Society 107(25): 7779-7780.
- Shinohara H (2000) Endohedral metallofullerenes. Reports on Progress in Physics 63(6): 843-892.
- Wudl F, Hirsch A, Khemani KC, Suzuki T, Allemand PM, et al. (1992) Survey of chemical reactivity of C60, electrophile and dieno-polarophile par excellence. Fullerenes, pp. 161-175.
- Chiang LY, Swirczewski JW, Hsu CS, Chowdhury SK, Cameron S, et al. (1992) Multi-hydroxy additions onto C60 fullerene molecules. Journal of the Chemical Society, Chemical Communications (24): 1791.
- Maggini M, Scorrano G, Prato M (1993) Addition of azomethine ylides to C60: Synthesis, characterization, and functionalization of fullerene pyrrolidines. Journal of the American Chemical Society 115(21): 9798-9799.
- Bingel C (1993) Cyclopropanierung von Fullerenen. Chemische Berichte 126(8): 1957-1959.
- Nelson MA, Domann FE, Bowden GT, Hooser SB, Fernando Q, et al. (1993) Effects of acute and subchronic exposure of topically applied fullerene extracts on the mouse skin. Toxicology and Industrial Health 9(4): 623-630.
- Scrivens WA, Tour JM, Creek KE, Pirisi L (1994) Synthesis of 14C-Labeled C60, its suspension in water, and its uptake by human keratinocytes. Journal of the American Chemical Society 116(10): 4517-4518.
- Sayes CM, Fortner JD, Guo W, Lyon D, Boyd AM, et al. (2004) The differential cytotoxicity of water-soluble fullerenes. Nano Letters 4(10): 1881-1887.
- Sayes CM, Gobin AM, Ausman KD, Mendez J, West JL, et al. (2005) Nano-C60 cytotoxicity is due to lipid peroxidation. Biomaterials 26(36): 7587-7595.
- Jia G, Wang H, Yan L, Wang X, Pei R, et al. (2005) Cytotoxicity of carbon nanomaterials: Single-wall nanotube, multi-wall nanotube, and fullerene. Environmental Science & Technology 39(5): 1378-1383.
- Lyon DY, Fortner JD, Sayes CM, Colvin VL, Hughes JB (2005) Bacterial cell association and antimicrobial activity of a C60 water suspension. Environ Toxicol Chem 24(11): 2757.
- Baati T, Bourasset F, Gharbi N, Njim L, Abderrabba M, et al. (2012) The prolongation of the lifespan of rats by repeated oral administration of [60] fullerene. Biomaterials 33(19): 4936-4946.
- Johnston HJ, Hutchison GR, Christensen FM, Aschberger K, Stone V (2009) The biological mechanisms and physicochemical characteristics responsible for driving fullerene toxicity. Toxicological Sciences 114(2): 162-182.
- Gu Y, Liu Y, Russell TP (2020) Fullerene-based interlayers for breaking energy barriers in organic solar cells. Chemplus Chem 85(4): 751-759.
- Jani M, Arcos-Pareja JA, Ni M (2020) Engineered zero-dimensional fullerene/carbon dots-polymer based nanocomposite membranes for wastewater treatment. Molecules 25(21): 4934.
- Dugan LL, Gabrielsen JK, Yu SP, Lin TS, Choi DW (1996) Buckminsterfullerenol free radical scavengers reduce excitotoxic and apoptotic death of cultured cortical neurons. Neurobiol Dis 3(2): 129-135.
- Saleem J, Wang L, Chen C (2018) Carbon-based nanomaterials for cancer therapy via targeting tumor microenvironment. Advanced Healthcare Materials 7(20): e1800525.
- Chen Z, Mao R, Liu Y (2012) Fullerenes for cancer diagnosis and therapy: preparation, biological and clinical perspectives. Current Drug Metabolism 13(8): 1035-1045.
- Anilkumar P, Lu F, Cao L, Luo G, Liu P, et al. (2011) Fullerenes for applications in biology and medicine. Curr Med Chem 18(14): 2045-2059.
- Shi J, Zhang H, Wang L, Li L, Wang H, et al. (2013) PEI-derivatized fullerene drug delivery using folate as a homing device targeting to tumor. Biomaterials 34(1): 251-261.
- Mashino T, Shimotohno K, Ikegami N, Nishikawa D, Okuda K, et al. (2005) Human immunodeficiency virus-reverse transcriptase inhibition and hepatitis C virus RNA-dependent RNA polymerase inhibition activities of fullerene derivatives. Bioorg Med Chem Lett 15(4): 1107-1109.
- Bolskar RD, Benedetto AF, Husebo LO, Price RE, Jackson EF, et al. (2003) First soluble M@C60 derivatives provide enhanced access to metallofullerenes and permit in vivo evaluation of Gd@C60[C(COOH)2]10 as a MRI contrast agent. J Am Chem Soc 125(18): 5471-5478.
- Demir E, Nedzvetsky VS, Ağca CA, Kirici M (2020) Pristine C60 Fullerene Nanoparticles Ameliorate Hyperglycemia-Induced Disturbances via Modulation of Apoptosis and Autophagy Flux. Neurochem Res 45: 2385-2397.
- Wu Y, Wang R, Wang Y, Gao J, Feng L, et al. (2019) Distinct impacts of fullerene on cognitive functions of dementia vs. non-dementia mice. Neurotox Res 36(4): 736-745.
- Đurašević S, Nikolić G, Todorović A, Drakulić D, Pejić S, et al. (2020) Effects of fullerene C60 supplementation on gut microbiota and glucose and lipid homeostasis in rats. Food and Chemical Toxicology 140: 111302.
- Kraevaya O, Novikov AV, Shestakov AF, Ershova E, Savinova E, et al. (2020) Water-soluble fullerene-based nanostructures with promising antiviral and myogenic activity. Chemical Communications.
- Liu S, Chen D, Li X, Guan M, Zhou Y, et al. (2020) Fullerene nanoparticles: A promising candidate for the alleviation of silicosis-associated pulmonary inflammation. Nanoscale.
© 2022 Yashwant Pathak. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






