- Submissions

Full Text
Novel Research in Sciences
The Influence of Inorganic Salts on the Phase Diagram and Separating Ability of Aqueous Biphasic System: Peg/Sodium Citrate-Water
Eldar MA* and Gunel SM
Department of Physics, Azerbaijan
*Corresponding author: Eldar MA, Department of Physics, Azerbaijan
Submission: October 20, 2021;Published: November 12, 2021
.jpg)
Volume9 Issue5November, 2021
Abstract
The purpose of the proposed research is to ensure that the value of the distinction is adjusted to each application by using various supplements to separate and clean up biological objects. The work presents the results of studies of the phase diagram of the biphasic system composed of Polyethylene Glycol (PEG)/ sodium citrate-water and the effect of inorganic salts (sodium sulfate, sodium carbonate, sodium nitrate, potassium sulfate, potassium chloride, potassium iodide, potassium bromide) on the separating ability of this biphasic system. Analysis of the data presented shows that the change in the parameters of the phase diagram and different values of the separating ability (for PEG- sodium citrate-water system n*=9,3) of the biphasic system, depending on the nature of the additives. The utilized inorganic salts change the structure of water clusters. Following the change, the biphasic system components relative hydrophobicity rises, which results in phase separation. The displacements of the binodal of the phase diagram (Figure 1) in the direction of the origin of coordinates, an increase in the area of the heterogeneous region of the diagram upon the introduction of the studied salts indicate that these salts have a structuring effect on the aqueous medium.
Keywords: Biphasic systems; Inorganic salts; PEG; Sodium citrate; Separating ability
Introduction
Aqueous biphasic systems were formed when two particular chemically different polymers
(e.g., dextran (dex) and Polyethylene Glycol (PEG)) were mixed at appropriate concentrations
in an aqueous solution, and the solution separated into two immiscible phases. One phase
is rich in one polymer, and the second phase is rich in the other polymer, as with water as a
solvent in both phases [1].
It should be noted that such incompatibility of components in a common solvent (in
water) could also be observed in the mixtures of one polymer with some inorganic and
organic salts [2].
Albertson et al. [3] systematic studies of various water-polymer two-phase systems led to
the emergence of a new universal, highly effective, gentle, cost-effective method of separation
and purification of a wide variety of biological materials [3].
Because the solvent in both phases of the studied systems is water (70-80%), proteins,
nucleic acids, viruses, cells, etc., can be introduced into such systems. Depending on their
characteristics and distribution conditions (nature and concentration of phase-forming
components, nature and concentration of additives, etc.), these biological objects are unevenly
distributed over coexisting phases without losing their intact properties. It should be
noticed that the method (method of separation) is also successfully used for the quantitative
assessment of the relative hydrophobicity of high molecular weight compounds, which could
not be determined previously [4].
The Aqueous Two-Phase System (ATPS) composed of PEG 6000
and Sodium Citrate (SC) has been proposed to recover the valuable
soluble proteins from tannery wastewater. Thus, the proposed
ATPS can serve as an alternative to the conventional precipitation
method to recover the soluble proteins from tannery wastewater
[5]. Aqueous biphasic systems were used during downstream
processing, mainly in biotechnological and chemical industries [6].
Aqueous biphasic extraction processes offer the potential for lowcost,
highly selective separations. This counter-current extraction
technique is involved in the selective partitioning of either dissolved
solute between two immiscible aqueous phases [7].
Aqueous biphasic systems have been successfully used to
detect veterinary drug residues in food, separation of precious
metals, sewage treatment, and a variety of other purposes [8]. The
practical application of Aqueous Two-Phase Systems (ATPS) to
extraction processes has been exploited for several years to recover
biological products [9]. A critical overview of the fundamental
thermodynamic properties related to forming aqueous two-phase
systems and their application to extraction and purification of
bioparticles was studied previously [9].
For describing aqueous biphasic systems, it is traditional to
study the phase diagram of the system (binodal curves, connecting
lines, separating ability, etc.). The properties of the aqueous
medium of the phases of a two-phase system and the nature of the
phase diagrams are affected by different factors like concentration
of phase-forming polymers, concentration and composition of
salts [6,10], their molecular weight, nature of the second phaseforming
component and solvent, temperature, the presence of low
molecular weight additives [11,12-14].
Studying the effect of various additives, in particular, inorganic
salts, on the water-polymer biphasic system is important since
additives of inorganic salts are widely used to regulate the
distribution of biological materials in these systems [15]. Similar
research was carried out for biphasic systems, e.g., dextran-PEG,
dextran-PVP, and dextran-ficoll [16,17]. The works presented
that the degree of influence of the addition of inorganic salts on
the conditions of phase separation in the biphasic systems under
consideration is associated with the position of the salt in the
lyotropic series of the ability of salts to precipitate proteins in
aqueous solutions [18]. However, it seems interesting to study the
effect of inorganic salts (sodium sulfate, sodium carbonate, sodium
nitrate, potassium sulfate, potassium chloride, potassium iodide,
and potassium bromide) on the separation of biphasic systems into
polymer-organic and salt-water phases.
In the presented work, the phase diagrams of the water-polymer
biphasic system PEG 6000-sodium citrate (C6H5O7Na3)-water and
the concentration effect of the sodium nitrate on the position of the
binodal, on the value of the separating ability of the system were
investigated. The PEGs with different molecular weights are widely
used polymers in Aqueous Two-Phase Systems (ATPS) due to their
low toxicity, low price, and low volatile nature [6].
Results and Discussions
For illustrating aqueous biphasic polymer systems, it is conventional [1] to investigate the phase diagram-binodal curves, where the weight vs concentrations of the phase-forming components, the tie line, its length and angle of inclination, separation capacity, etc., are plotted along the coordinate axes. Figure 1 shows the binodal curve of the PEG (6000)-sodium citrate/water tie line, which is defined based on the method of least squares equation.
Figure 1: The influence of NaNO3 on the phase diagram of aqueous biphasic systems PEG-sodium citrate.
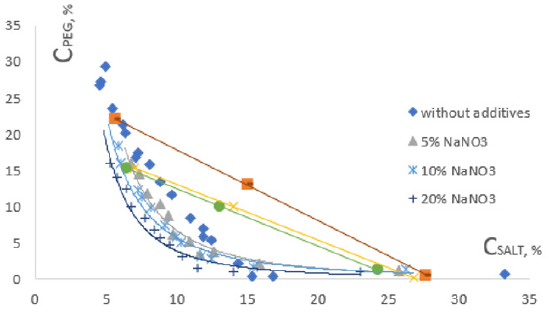
The binodal curve and tendency angle of connecting (or tie) lines are taken as the main characteristic of polymer-polymer-water two-phase systems. The phase diagrams depend on many factors: the nature of polymers, their molecular weights, temperature, the presence of low molecular weight additives, etc. [16].
Figures 2, 3 and Table 1 show the experimental results describing the binodal and tie lines of the phase diagram of the studied biphasic system in the absence and presence of various salts, where the concentrations of the phase-forming components are plotted along the coordinate axes. The curves (binodal) delimit the region of existence of homogeneous solutions (under the binodal) and the region of existence of heterogeneous (above the binodal) solutions. Figure 3 describes a change in the position of binodal in the presence of NaNO3 at different concentrations.
Table 1: Changes of the critical point of the binodal of the PEG (6000) - C6H5O7Na3-H2O system with varying NaNO3 concentrations.
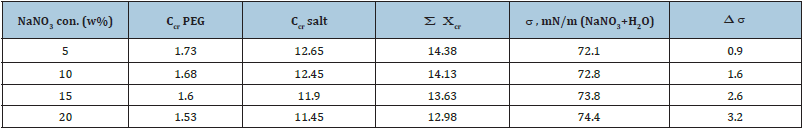
Figure 2: Binodal curve and tie lines of the biphasic system PEG/sodium citrate -H2O.
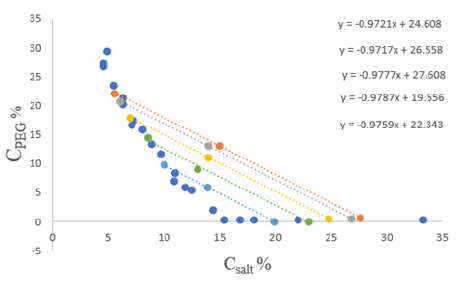
Figure 3: The influence of salts (NaNO3, Na2CO3, Na2SO4) on the formation of biphasic systems PEG-C6H5O7Na3-H2O.
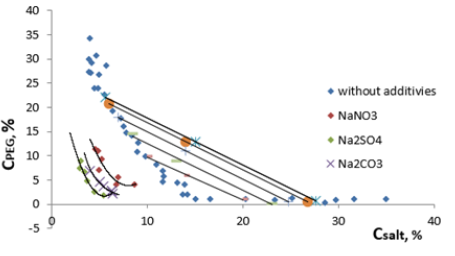
As it follows from the results obtained by adding all salts to the
system, the binodal are somewhat displaced towards the origin
of coordinates extensively when the salt concentration increases
(Figure 3). Proportionally the area of the heterogeneous region
of the phase diagram increases. The variations in the system with
two phases occur at lower concentrations of the phase-forming components, which indicates that the structuring of the aqueous
medium of the system takes place under the influence of added
salts. The structuring of the aqueous medium of the biphasic
system phases is due to changes in the degree of hydration of
the phase-forming components. Therefore, the differences in the
relative hydrophobic properties of the phases increase, leading to
worse compatibility of these components in the common solvent,
and naturally, to separating the system into two phases at lower
concentrations the phase-forming components.
Figure 4 shows the data describing the concentration effect
of sodium nitrate on the total concentration of phase-forming
components at the critical point of the studied biphasic system
(PEG and sodium citrate). As shown in Figure 4, with an increase in
the concentration of the added salt (NaNO3), the total concentration
of phase-forming components decreases at the critical point for
separating the system into two phases.
Figure 4: Dependence of total concentration of phaseforming components from the concentration of sodium nitrate.
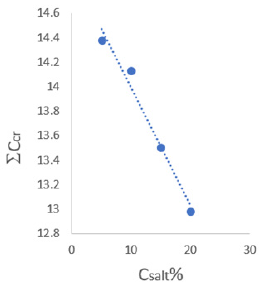
Figure 5 shows changes in the surface tension of water (Δσ)
depending on the concentration of salt (NaNO3) and based on the
data in Figure 4 and 5. The dependence of the shift of the value of
the total concentration  of the phase-forming components of
the biphasic system on the surface tension (Figure 6).
of the phase-forming components of
the biphasic system on the surface tension (Figure 6).
Figure 5: Changes in the surface tension of water (Δ σ) depending on the concentration of NaNO3.
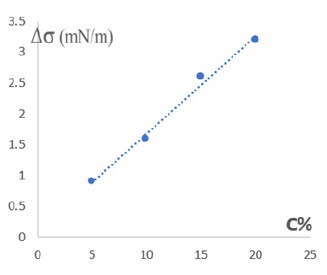
Figure 6: Dependence of total concentration of the
phase-forming components 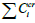 from Δ σ.
from Δ σ.
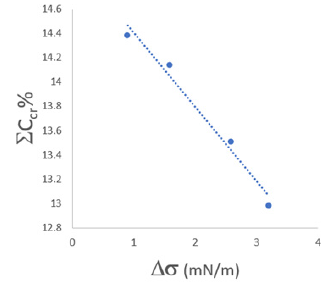
Figure 6 describes the magnitude of the shift in the total
concentration of the system components under the influence of
the added salt (NaNO3) and its effect on the surface tension of pure
water. There is a good correlation indicating the structuring of
water under the influence of the introduced salt into the system.
For a more detailed analysis of the results obtained, let us
consider the mechanism of the effect of salts on water based on
Samoilov’s theory [19]. According to this theory, an ion (cation
or anion) destroying the structure of water means an ion, in the
vicinity of which water molecules exchange with molecules of
“free” water in the volume with a higher frequency than molecules
of “free” water among themselves, i.e.
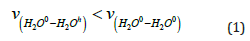
Where 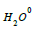 - water in volume,
- water in volume, 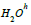 - water in the hydration
shell of the ion. In other words, the settled life of a water molecule
near a water ion in the volume
- water in the hydration
shell of the ion. In other words, the settled life of a water molecule
near a water ion in the volume
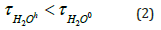
and an ion that stabilizes the structure of water means ions for which the following conditions are met:
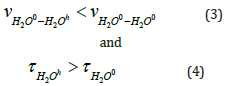
Our results indicate that in our case, conditions (3) and (4)
hold. When salts are introduced into the system, water molecules of
anions (all studied salts have the same cations) exchange with “free water” molecules at a lower frequency than free water molecules
with each other, which ultimately leads to the structuring of all
water molecules [20].
All the above discussion makes it possible to qualitatively
explain the results obtained in this work on the effect of salt
additions on the characteristics of the phase diagram of the PEG/
sodium citrate-water|.
Thus, the displacement of the binodal of the phase diagram
towards the origin of coordinates, an increase in the area of the
heterogeneous region of the diagram upon the introduction of the
studied salts (NaNO3, Na2SO4, Na2CO3, KCl, KBr, KJ, K2SO4), as well as
a decrease in the total concentration of phase-forming components
at the critical point of the two-phase system with an increase in
salt concentration (for example, NaNO3) clearly indicates that these
salts have a structuring effect on the aquatic environment of the
system.
It should be emphasized that changes in the characteristics
of an aqueous biphasic system naturally affect the distribution of
various substances in these biphasic systems. To quantitatively
characterize the difference in the affinity of the phases to the
distributing substance, we studied the separating ability (n*) of the
biphasic PEG (6000)-sodium citrate-water system at T = 298.15K
in the absence and presence of various additives. The obtained
data are presented in Table 2. The separating ability of the system
is determined by the method proposed in work [4]. The results
obtained show that the addition of urea to the aqueous biphasic
system reduces its separation capacity (n* = 5.20 in the presence
of 1.25mol/l of urea, while n* = 6.5 in the absence of additives). In
contrast, the addition of carbohydrates, such as glucose and sucrose
to the system leads to an increase in the separation capacity of the
system.
Table 2: Separating ability PEG - sodium citrate- water in the presence of some salts.

Urea changes the value (n*) associated with the destruction of
the water structure in the corresponding phases. Urea also leads to
an increase in PEG hydration and a change in the latter molecule
nearest the aqueous environment, which boosts the compatibility of
the system components. This contributes to the convergence of the
properties of the phases, consequently, a more uniform distribution
of substances between the two phases and leads to a decrease
in the separation capacity. With the addition of carbohydrates,
the structuring of the aqueous environment takes place, which
should lead to a decrease in PEG hydration. Eventually, it leads to
a deterioration of hydrophobic phases. The distribution coefficient
can be elevated via using mineral salts results in an increase in the
separation capacity of the system [21-23].
A significant increase in the separating ability of the investigated
biphasic system with the introduction of salts (Table 2) indicates
that these salts very strongly stabilize the structures of the aqueous
medium in the phases of the aqueous biphasic system.
Conclusion
The binodal curves for PEG 6000 + sodium citrate + water
system at 298.15K were constructed and adequately fitted with a
non-linear equation. The least-squares method was used to define
the tendency angle of the tie line. With the influence of salts (sodium
sulfate, sodium carbonate, sodium nitrate, potassium sulfate,
potassium chloride, potassium iodide, potassium bromide), the
obtained binodal curve was slipped to the beginning of coordinate
at the low concentrations. Two-phase systems occurred with a low
concentration of components (PEG and salt) at a low concentration
of polymer and salt, which formed phases. To quantitatively
characterize the difference in the affinity of the phases to the
distributing substance, we studied the separating ability (n*) of the
biphasic PEG (6000) -sodium citrate-water system at T = 298.15K
in the absence and presence of various additives. With the addition
of sodium sulfate (n* = 14.6 in the presence of 2.36mol/l of sodium
sulfate, while n* = 9.3 in the absence of additives), the structuring
of the aqueous environment took place, which led to a decrease
in PEG hydration and deterioration in hydrophobic phases. An
increase in the distribution coefficient increases the separation
capacity of the system. The effect of salts on the binodal curve and
significant increase in the separating ability of the investigated
biphasic system indicates that these salts (sodium sulfate, sodium
carbonate, sodium nitrate, potassium sulfate, potassium chloride,
potassium iodide, potassium bromide) very strongly stabilize the
structures of the aqueous medium in the phases of the aqueous
biphasic system.
The studied systems can create conditions that enable the
separation and extraction of various biological objects, which
promises potential application in biotechnology and pharmacology.
Conflicts of Interest
There are no conflicts to declare.
References
- Albertson PP (1970) Partition of cell particles and macromolecules in polymer two-phase systems. Adv Protein Chem 24: 309-341.
- Masimov EA, Ismailov EH, Odzhaqverdiyeva SY (2015) Complexation of polyethylene-glycol with the sodium salts of citric and succinic acids in the aqueous solutions. studies by dynamic light scattering and uv/vis spectrophotometry. Journal of Advances in Chemistry 11(8): 3866-3872.
- Albertson PA (1958) Biochim Biophys Acta 2: 378-394
- Zaslavsky BY, Masimov EA, Boche G, Kaupp G, Rabinovitz M (1988) Methods of analysis of the relative hydrophobicity of biological solutes. In: Physical Organic Chemistry. Topics in Current Chemistry 146. Springer, Berlin.
- Raja S, Murty VR (2013) Optimization of aqueous two-phase systems for the recovery of soluble proteins from tannery wastewater using response surface methodology. Journal of Engineering.
- Mazzola PG, Lopes AM, Hasmann FA, Jozala AF, Penna TC, et al. (2008) Liquid-liquid extraction of biomolecules: an overview and update of the main techniques. J Chem Technol Biot 83(2): 143-157.
- Chaiko DJ, Zaslavsky B, Rollins AN, Vojta Y, Gartelmann, J, et al. (1996) Metal separations using aqueous biphasic partitioning systems. United States.
- Iqbal M, Tao Y, Xie S, Zhu Y, Chen D, et al. (2016) Aqueous Two-Phase System (ATPS): An overview and advances in its applications. Biol Proced Online 18.
- Da Silva LHM, Loh W (2006) Aqueous two-phase systems: Fundamentals and applications for partitioning/purification of proteins. Química Nova 29(6): 1345-1351.
- Moattar ZMT, Hamzehzadeh S, Nasiri S (2012) A new aqueous biphasic system containing polypropylene glycol and a water-miscible ionic liquid. Biotechnol Progr 28: 146-156.
- Barani A, Pirdashti M, Heidari Z, Dragoi EN (2018) Influence of the molecular weight of polymer, temperature and pH on phase diagrams of poly (ethylene glycol) + di-potassium tartrate aqueous two-phase systems. Fluid Phase Equilib 459: 1-9.
- Barbosa AA, Bonomo RCF, Martins CV, Fontan RCI, Júnior ECS, et al. (2016) Equilibrium data and physical properties of aqueous two-phase systems formed by PEG (1500 and 4000) g·mol-1 + sodium sulfate + water at different temperatures and pH 2. J Chem Eng Data 61(1): 3-11.
- Han J, Wang Y, Yu C, Li Y, Kang W, et al. (2012) (Liquid + liquid) equilibrium of (imidazolium ionic liquids + organic salts) aqueous two-phase systems at T = 298.15 K and the influence of salts and ionic liquids on the phase separation. J Chem Thermodyn 45(1): 59-67.
- Liu Y, Wu Z, Zhao Y (2015) Liquid-liquid equilibrium correlation of aqueous two-phase systems composed of polyethylene glycol and nonionic surfactant. Thermodynamica Acta 602: 78-86.
- Ferreira LA, Teixeira JA (2011) Salt effect on the aqueous two-phase system peg 8000-sodium sulfate. J Chem Eng Data 56(1): 133-137.
- Zaslavsky BY, Bagirov TO, Borovskaya AA, Gasanova GZ, Gulaeva ND, et al. (1986) Aqueous biphasic systems formed by nonionic polymers I. Effects of inorganic salts on phase separation. Colloid Polymer Sci 264: 1066-1071.
- Zaslavsky BY (1987) BMC 296(2): 98-101.
- Zhang K, Su T, Cheng F, Lin Y, Zhou M, et al. (2020) Effect of sodium citrate/polyethlene glycol on plasticization and retrogradation of maize stratch. International Journal of Biological Macromolecules 154: 1471-1477.
- Samoilov OY (1967) Structure of water solutions of electrolytes. pp. 15-18.
- (2018) Journal of Baku Engineering University 2(2): 71-76.
- Masimov EA, Hasanov AA, Hasanova HT (2015) International Journal of Applied and Fundamental Research 4(1): 40-44.
- Zaslavsky BY, Masimov EA (1986) 288(1).
- Raja S, Murty VR (2013) Liquid-liquid equilibrium of poly (Ethylene Glycol) 6000 + Sodium Succinate + Water System at different temperatures. The Scientific World Journal 2013: ID 819259.
© 2021 Eldar MA. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






