- Submissions

Full Text
Novel Research in Sciences
Cervicogenic Headache: A New Perspective
Rob Sillevis* and Valerie Weiss
Department of Rehabilitation Sciences, Florida Gulf Coast University, Fort Myers, Florida, United States
*Corresponding author: Rob Sillevis, Department of Rehabilitation Sciences, Florida Gulf Coast University, 10501 FGCU Boulevard South, Marieb 428, Fort Myers, Florida 33965, Florida, United States
Submission: August 14, 2021;Published: September 2, 2021
.jpg)
Volume9 Issue3August, 2021
Mini Review
Headaches are common and result in a significant societal burden. Approximately 50% of the adult population in the Unites states is affected by headaches. Headaches result in a significant number of sick days and thus result in a loss of overall productivity. Currently, around 4% of the population experiences Cervicogenic Headaches (CGH) [1,2]. The prevalence of CGH is about 15% of all headaches [3]. Cervicogenic headaches are defined as “headache caused by a disorder of the cervical spine and its component bony, disc and/or soft tissue elements, usually but not invariably accompanied by neck pain” by the International Headache Society [4]. Individuals with CGH are typically managed by a variety of providers, such as general practitioners, neurologists, interventional pain specialists, chiropractors, and physical therapists. The exact underlying mechanism that results in CGH remains elusive. Despite the fact that there is no definite causation, individuals with CGH are likely to be treated with both pharmacological and non-pharmacological interventions. Considering the exact causative mechanism for CGH remains unclear the current management strategies and their long-term effects have to be called in question. The most current common rationale explaining the origination of CGH is based on referred pain from the upper cervical spine. Although any structure in the upper cervical spine could be considered causative, it appears that the Atlanto-Axial joint (AA) and the C2-3 zygapophyseal joint are the most frequent sources of CGH. Anatomical dissections demonstrate a direct relationship between the trigeminal nerve and spinal nerves C1 through C3 at the trigeminocervical nucleus: [5,6] the dorsal horns of the upper cervical levels synapse within the trigeminocervical nuclei. This pathway would allow nociceptive signals from the cervical spine to converge with the secondorder trigeminal neuron. Furthermore, this neurogenic route could affect the ophthalmic branch of the trigeminal nerve and thus explain the subjective perception of orbital pain in subjects with CGH [5-7].
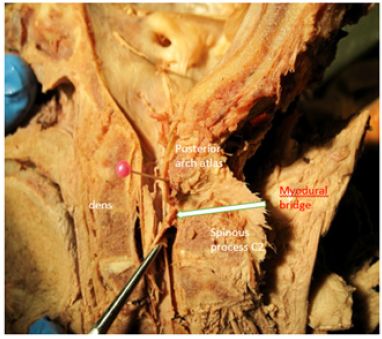
Another rationale for developing CGH might be directly related to the muscles that move the upper cervical region. Thus, muscular dysfunctions have been identified as a possible contributing factor for the development of CGH. Humans typically function in the upright position, and based on this, the upper cervical spine muscles will have to maintain the head position against the vertical gravitational compressive force. This need for muscle control commonly leads to increased muscle tone in the posterior upper cervical spine and the development of trigger points. It has been demonstrated that trigger points in the cervical musculature can refer back to the head and face [8,9]. More recently, collagenous tissue connections between the nuchal ligament and the dura mater have been demonstrated [10]. This connection implies a direct mechanical relationship between the cervical spine and the dura mater. Likewise, fascial connections exist between the Rectus capitis posterior minor, Rectus capitis posterior major, and the Obliquus capitis inferior with the surrounding dura [11,12]. These connections between muscle and dura have been identified as “myodural bridges,” spanning the arches of C1 and C2 and blending with the meningovertebral ligaments. The myodural bridges expand from there to cross the epidural space and connect into the posterior aspect of the dura (Figure 1) [11,12]. One could wonder about this anatomical significance of the connection between ligament, muscles, and the dura mater. This interrelatedness of structures might explain why clinicians have identified positive neural tension testing when treating individuals with cervicogenic headaches [13-15].
Figure 1:Midsagittal dissection demonstrating the myodural bridge between C1-2. White arrow indicates the dural connection.
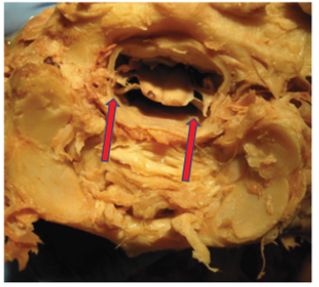
Functional movement of the upper cervical spine is dependent on musculature, cervical joints, and stabilizing ligaments. The atlas plays a pivotal role in the rotational motion pattern of the upper cervical region. Hence, the position of the atlas relative to the axis appears to correlate with the presence of CGH as well [16,17]. Under normal circumstances, the atlas is well stabilized, relative to the dens of the axis, by the transverse ligament, not causing side effects. Of note, the transverse ligament has been identified as the strongest ligament in the region [18]. Mechanically, the transverse ligament allows the atlas to move in the transverse plane around the odontoid process. This rotational movement of the atlas is about 40-45 degrees relatively to the axis, contributing to about 50% of the total rotation of neck [19]. The suboccipital muscles, especially contraction of the homolateral obliquus capitis superior and inferior muscles, will result in rotation of the atlas relatively to the axis. It has been previously proposed and demonstrated that extended contraction of the homolateral obliquus capitis superior and inferior muscles can result in rotation of the atlas in a positional default position of the atlas [16,17]. (Figures 2 & 3) demonstrate such rotation of atlas performed manually on a dissected C0-C1 segment in the transverse plane. It can be noted that there is a significant shift of the spinal cord within the dural sac toward the side of rotation. When measuring this shift of the spinal cord to the rotational side compared to the neutral position (Figure 4), it was identical for both directions. Therefore, we postulate that this shift is significant and that as a result of ongoing unilateral muscular tone of the obliquus capitis inferior and superior muscles, there is tensioning of the dura. Kumar [20] identified that the cervical dura does not have a rich innervation of pain-sensitive nerve fibers as opposed to the cranial dura which does have pain-sensitive fibers. This is supported by the findings of Witten [21], who demonstrated that the cranial dura plays a contributing role in the development of headaches. Typically, subjects with CGH report headaches starting in the upper dorsal neck region (possibly due to prolonged muscle hypertonicity) and then spreading to the head. Jansen [22] demonstrated that after a cervical dorsal laminectomy freeing the dura from compression and or irritation, the subjects were relieved from CGH. Noseda [23] suggest that the upper cervical muscle tone could result in dural tensioning with associated pain. They further demonstrated that the posterior dura overlying the cerebellum is innervated cervicovasular neurons, and this could lead to sensitization and pain of this region. In conclusion, there are various causes for cervicogenic headaches, including referred pain, muscular dysfunction, myodural bridges, and instability of the atlas. Moreover, there appears to be emerging evidence to consider the dura mater as a key component in the pathogenesis of cervicogenic headaches. Future research should further investigate the relationship between the dura mater and CGH as well as the position of the atlas related to dural tensioning.
Figure 2: Left rotation of atlas. The red arrow represents the movement of atlas to the left, relative to atlas and tension can be seen in the tissues on the right in the vertebra canal.

Figure 3:Right rotation of atlas. The red arrow represents the movement of atlas to the right relative to atlas and tension can be seen in the tissues on the left in the vertebra canal.
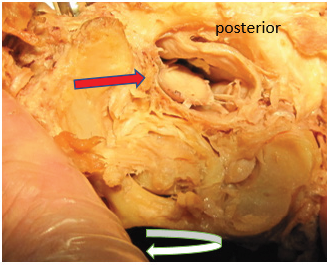
Figure 4: Neutral position of atlas and it can be seen that the spinal cord is symmetrical positioned in the dural sack. Red arrows indicate this position.
References
- Sjaastad O, Bakketeig L (2008) Tension-type headache: Comparison with migraine without aura and cervicogenic headache. The Vaga study of headache epidemiology. Functional Neurology 23(2): 71-76.
- Sjaastad O, Bakketeig LS (2008) Prevelance of cervicogenic headache: Vaga study of headache epidemiology. Acta Neurol Scand 117(3): 173-180.
- Nilsson N (1995) The prevalence of cervicogenic headache in a random population sample of 20-59 year olds. Spine 20(17): 1884-1888.
- Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, (3rd), Cephalalgia 38(1): 1-211.
- Hall T, Briffa K, Hopper D (2008) Clinical evaluation of cervicogenic headache: A clinical perspective. J Man Manip Ther 16(2): 73-80.
- Biondi DM (2001) Cervicogenic headache: Diagnostic evaluation and treatment strategies. Curr Pain Headache Rep 5(4): 361-368.
- Bogduk N (2014) The neck and headaches. Neurol Clin 22(1): 151-171.
- Bodes-Pardo G, Pecos-Martin D, Gallego-Izquierdo T, Salom-Moreno J, Fernandez-de-Las-Penas C, et al. (2013) Manual treatment for cervicogenic headache and active trigger point in the sternocleidomastoid muscle: A pilot randomized clinical trial. J Manipulative Physiol Ther 36(7): 403-411.
- Fernandez-de-Las-Penas C, Ge HY, Alonso-Blanco C, Gonzalez-Iglesias J, Arendt-Nielsen L (2010) Referred pain areas of active myofascial trigger points in head, neck, and shoulder muscles, in chronic tension type headache. J Bodyw Mov Ther 14(4): 391-396.
- Sillevis R, Hogg R (2020) Anatomy and clinical relevance of sub occipital soft tissue connections with the dura mater in the upper cervical spine. Peer J 8.
- Scali F, Marsili ES, Pontell ME (2011) Anatomical connection between the rectus capitis posterior major and the dura mater. Spine (Phila Pa 1976) 36(25): 1612-1614.
- Pontell ME, Scali F, Marshall E, Enix D (2013) The obliquus capitis inferior myodural bridge. Clin Anat 26(4): 450-454.
- Szikszay TM, Luedtke K, Harry von P (2018) Increased mechanosensivity of the greater occipital nerve in subjects with side-dominant head and neck pain-a diagnostic case-control study. J Man Manip Ther 26(4): 237-248.
- Caamano-Barrios LH, Galan-Del-Rio F, Fernandez-de-Las-Penas C, Cleland JA, Plaza-Manzano G, et al. (2019) Evaluation of neurodynamic responses in women with frequent episodic tension type headache. Musculoskelet Sci Pract 44.
- Piekartz HJM, Schouten S, Aufdemkampe G (2007) Neurodynamic responses in children with migraine or cervicogenic headache versus a control group. A comparative study Man Ther 12(2): 153-160.
- Sillevis R, Wyss K (2015) The management of a positional default of atlas in a patient with cervicogenic headache: A case report. Clin Case Rep Rev 1(10): 218-223.
- Sillevis R, Swanick K (2019) Musculoskeletal ultrasound imaging and clinical reasoning in the management of a patient with cervicogenic headache: A case report. Physiother Theory Pract, pp. 1-11.
- Mantripragada S, Kannivelu A, Peh WC (2020) Magnetic resonance imaging of cervical ligamentous anatomy and traumatic ligamentous injuries. J Med Imaging Radiat Oncol 64(3): 368-376.
- Wu SK, Kuo LC, Lan HC, Tsai SW, Su FC (2010) Segmental percentage contributions of cervical spine during different motion ranges of flexion and extension. J Spinal Disord Tech 23(4): 278-284.
- Kumar R, Berger RJ, Dunsker SB, Keller JT (1996) Innervation of the spinal dura. Myth or reality? Spine (Phila Pa 1976) 21(1):18-26.
- Witten A, Marotta D, Cohen-Gadol A (2021) Developmental innervation of cranial dura mater and migraine headache: A narrative literature review. Headache 61(4): 569-575.
- Jansen J (1999) Laminoplasty-a possible treatment for cervicogenic headache? Some ideas on the trigger mechanism of CeH. Funct Neurol 14(3): 163-165.
- Noseda R, Melo-Carrillo A, Nir RR, Strassman AM, Burstein R (2019) Non-trigeminal nociceptive innervation of the posterior dura: Implications to occipital headache. J Neurosci 39(10):1867-1880.
© 2021 Rob Sillevis. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






