- Submissions

Full Text
Novel Research in Sciences
Satellite Glial Cells: Their Morphology and Role as Modulators of Sensory Input
David John Mackay Smith*
Skin cancer institute, 10 Mary Street, NOOSAVILLE, QLD, Australia
*Corresponding author: David John Mackay Smith, Skin cancer institute, 10 Mary Street, NOOSAVILLE, QLD, Australia
Submission: July 26, 2021;Published: August 27, 2021
.jpg)
Volume9 Issue2August, 2021
Abstract
Satellite Glial Cells (SGCs) are found in sensory and autonomic ganglia of the peripheral nervous system. They have a unique relationship with the nerve cell bodies, somata, completely enveloping them in a thin sheet, mostly individually, within the ganglia. The gap between somata and these glia mimics the synaptic cleft allowing bi-directional communication. The SGCs are responsible for neuron homeostasis but nerve injury or inflammation cause SGC activation often with augmentation of neuronal activity and in some situations contributing to pain syndromes.
Abbreviations:DRG: Dorsal Root Ganglia; TG: Trigeminal Ganglia; SGCs: Satellite Glial Cells; GFAP: Glial Fibrillary Acid Protein; VGCC: Voltage-Gated Calcium Channels; ER: Endoplasmic Reticulum; DLK: Dual Leucine Zipper-Bearing Kinase; HDAC5: Histone Deacetylase 5
Introduction
Figure 1:Location and morphology of SGCs within the peripheral nervous system.
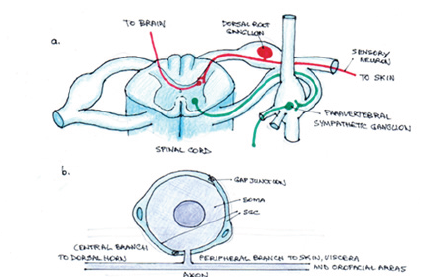
Dorsal Root Ganglia (DRG) and Trigeminal Ganglia (TG) neurons are the primary afferents transmitting sensory information from the periphery to the central nervous system. DRGs are situated on posterior roots of spinal nerves, TGs are on the cerebral surface of the temporal bone. Neurons in these ganglia have unique pseudo-unipolar structure (Figure 1). The cell body (soma) of each neuron gives out one axon that bifurcates into two branches. The peripheral branch travels to skin, viscera and orofacial areas. The central branch travels to the dorsal horn of the spinal cord or the medullary dorsal horns of the trigeminal spinal tract nucleus. Afferent spikes to somata provides information for protein synthesis and maintenance of optimal levels of receptors and ion channels in both nerve terminals. Activity in somata dynamically contributes to nerve signaling to dorsal horn cells. Non-neuronal cells also affect neuronal activity through glial-neuron interaction. Somata of sensory neurons do not form synaptic contacts with one another in the ganglia, instead they are enveloped by the SGCs.
a. SGCs surround individual cell bodies (somata) of sensory and autonomic neurons within sensory, sympathetic and parasympathetic ganglia. The position of the DRG within the sensory pathway from skin to brain and a paravertebral sympathetic ganglion is also indicated.
b. A soma with two surrounding SGCs showing pseudo-unipolar arrangement of the axon and the SGCs linked by gap junctions.
Satellite glial cells envelop the somata of peripheral sensory and autonomic nerves. Located in sensory, sympathetic and parasympathetic ganglia where they form a thin, tight sheath around each individual neural soma. A narrow gap, the approximate width of a synaptic cleft (20nm) between the neuronal stoma and the SGC enables control of the extracellular space and allows rapid bidirectional communication. SGCs are homeostatic cells supporting and modulating the activity of sensory neurons by expression of glutamate transporters and enzymes involved in the glutamate-glutamine cycle and buffering potassium to maintain an appropriate K+ concentration surrounding the neuron. SGCs express neurotransmitters, receptors, transporters and ion channels. They release neurotransmitters and neuroactive substances such as ATP and cytokines as part of glia-neuron signaling. In their naïve state they are most similar to astrocytes, particularly in relation to glutamate and K+ control, but are also very similar to Schwann cells, both being of neural crest origin. A range of neuronal stressors triggers their activation. Upregulation of Glial Fibrillary Acid Protein (GFAP) is used as a marker for activation. As compared to other cells in the DRG, SGCs are enriched in genes related to the immune system and cell to cell communication.
The Glia
SGCs are similar to CNS astrocytes and oligodendrocytes and represent an intermediate type but the CNS glia are highly branched whereas the SGCs are not and generally do not myelinate. They all share molecular markers and like astrocytes, SGCs express glutamine synthetase, S100, vimentin, Glutamate Aspartate Transporter (GLAST), Connexin 43(Cx43), and Kir 4.1 potassium channels. SGCs also express the transcription factor Sox2 which is typical of stem cells [1], and as such, can function as progenitors, giving rise to neurons [2,3]. All glial cells in the peripheral nervous system are of neural crest origin. SGCs contain cadherin 19 which is a Schwann cell marker, and it has been proposed that SGCs represent a subpopulation of cells of Schwann cell lineage whose differentiation has been arrested due to contact with sensory neuron soma [4,5].
Glial activation-gliosis
Upon activation, glial cells show an immune cell-like response, manifesting increased cytokine production, behaving as antigenpresenting cells and can be involved in active phagocytosis. SGCs express macrophage markers MHC type II, CD40 and CD54, which represents a unique leukocyte phenotype [6]. The hallmark of both astrocyte and SGC activation is upregulation of GFAP. Activation also induces stronger gap junction-mediated SGC-SGC and neuron-SGC coupling, increased sensitivity to ATP, down regulation of Kir4.1 potassium channel and increased pro-inflammatory cytokine synthesis and release (IL-1β, IL-6, TNF and fractalkine) which can act on neurons and increase their excitability and firing.
Features of SGCs
Ion channels: The main K+ channel is Kir 4.1 which can regulate neuronal activity. Injury suppresses Kir 4.1 function. Reduced Kir4.1 permeability depolarises the SGC, inducing release of excitatory mediators, such as ATP. Silencing Kir4.1 in the TG leads to pain-like behaviour [7]. SGCs do not have voltage-dependent Na+ or Ca2+channels and therefore cannot conduct action potentials.
Gap junctions: Both CNS and peripheral glia express gap junction channels, which provide a pathway for diffusion of ions and small molecules. The most abundant connexin (Cx), a gap junction protein, in mouse DRG and TG is Cx43 and Cx32. Cx43 is upregulated following nerve injury or inflammation. There is evidence for increased coupling by gap junctions amongst SGCs in a wide range of pain models. Coupling not only increases within sheaths surrounding individual neurons but extends to sheaths surrounding adjacent neurons via newly formed processes (Figure 2).
Figure 2: Gap junctions in intercellular communication.

a. Under normal conditions, SGCs surrounding each neuron (N1, N2) individually and are coupled together by gap junctions. b. When neuron 1 is activated, K+ is released which sustains neuronal depolarization and activates Panx1 to release signaling molecules including ATP. ATP binds to P2X7 receptors located only on SGCs but other P2X ionotropic and P2Y metabotropic receptors located on both neurons and SGCs provide positive feedback. Gap junctions between SGCs of individual neurons are strengthened with new gap junctions between SGCs of different neurons, between SGCs and Neurons, and between neurons can be formed.
Pannexins: Pannexins (Panx) are homologues of gap junction proteins of invertebrates. Although Panx1 does not form gap junctions, it does form membrane channels that allow the release of ATP. Panx1 expression is upregulated in a number of pain models, suggesting a role for Panx1-mediated ATP release in nociception. Analgesic effect was obtained in mice with glial targeted but not neuron specific Panx1 deletion, indicating the dominant role of SGCs in ATP release [8].
Receptors: Purinergic transmission is a form of extracellular signaling mediated by purine nucleotides, such as ATP, which activates purinergic receptors. In the DRGs, ecto-ATPases, which break down ATP to ADP, are found in Schwann cells and SGCs, but not neurons. ATP is a messenger in neuron-glial interactions and in the pain pathways. Cells release ATP by vesicular or channel mediated mechanisms such as P2X7R or Panx1 channels. This enables Glial cells to regulate ATP level in the ganglion.
Figure 3:Neuron-SGC bidirectional communication.
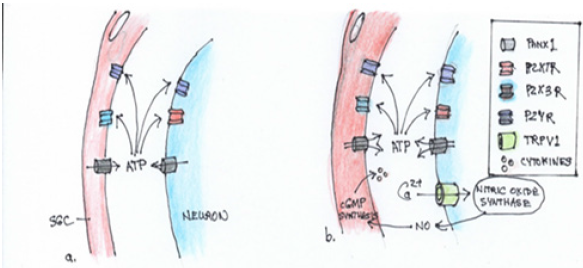
Nitrous oxide: Nitrous Oxide (NO), because of ease of diffusion across the narrow gap between SGC and neuron, has a role as an intercellular messenger. NO synthesis in DRG neurons increases after peripheral inflammation and NO induces SGC activation through Cyclic Guanosine Monophosphate (cGMP) production leading to GFAP upregulation, which contributes to neuronal excitation. There is evidence that NO plays a major role neuron- SGC communication. Functional consequences remain obscure but abnormal neuronal activity in sensory ganglia contributes to chronic pain [9]; Figure 3.
a. Resting condition.
b. Neuronal excitation and SGC activation.
The neuronal cell body fires at a higher rate of action potentials e.g. Neurotrauma. Increased intracellular calcium activates nitric oxide synthase to produce nitric oxide which diffuses to the SGC where it induces Cyclic Guanosine 5’-Monophosphate (cGMP) synthesis, activating the SGC. SGC activation stimulates release of ATP and cytokines, gap junction formation and increased sensitivity to ATP. Ca2+, calcium; NO, nitric oxide; P2R, purinergic receptor: Panx1, pannexin 1; TRPV1, transient receptor potential vanilloid type 1 channel.
Gap junctions, pannexins and pain
Studies exploring gap junction expression and dye coupling changes in sensory ganglia showed that gap junctions between SGCs surrounding individual somata and between SGC sheaths of neighbouring neurons were increased with a wide range of pain models [10]. There is good evidence that increased gap junctions and Panx1 expression seen in sensory ganglia in chronic pain models is responsible for peripheral sensitization. Peripheral activity drives central sensitization in pain states [11]. There is also evidence that gap junctions in the spinal cord play a role in chronic pain. Targeted blockade of spinal cord Panx1 might be effective for pain relief, although Panx1 expression in the dorsal horn was not changed in this pain model making it less likely that Panx1 is a key component of central sensitization [12]. Injury enhancing both Cx43 and Panx1 activity is a consequence of increased extracellular K+ and intracellular Ca2+, contributing to excitatory drive and contributing to allodynia. Changes are less well understood in the dorsal horn but it is likely similar mechanisms operate at the point of convergence of peripheral sensory information.
SGC response to axonal injury
There are changes in the transcriptional fingerprint of SGCs following peripheral nerve injury. SGCs are enriched in genes related to immune systems and cell-cell communication with a different response between day 3 to day 14, the two investigated time points, indicating dynamic modulation of SGC function over time. There is down regulation of a significant number of genes related to cholesterol synthesis at both day 3 and day 14. Particularly, the gene encoding the rate limiting enzyme Hmgcs1 responsible for HMG-CoA production in the cholesterol biosynthetic cascade. Reduced cholesterol synthesis by SGCs contributes to lowering of cholesterol levels in injured sensory neurons with the purpose of promoting neuronal repair and axonal regeneration, however at the price of increasing nociceptive sensitivity [13]. There is also up regulation of genes related to the immune system, MHC protein complexes and leukocyte migration e.g. Gene ccl 2 encoding chemokine monocyte chemoattractant protein 1(MCP1) is upregulated by day 3 and persisting to day 14. These cytokines are responsible for recruitment of macrophages. SGC up regulation of MHC II after peripheral nerve injury affects the activation state of T cells, modulating immune response. After injury, macrophages can be found in closer proximity to DRG neurons, the macrocytes surrounding the somata in an SGC-like manner. Macrocyte-neuron interactions have been suggested to be critical for peripheral sensitization and neuropathic pain [14].
Axon-soma communication in neuronal injury
Extensive lengths of neuronal processes necessitate efficient mechanisms for communication within the cell body. Early events are rapidly encoded by changes in ion fluxes. A calcium wave propagates from the lesion site. Increased calcium concentration at the site is required for many aspects of repair, retrograde signaling, cytoskeletal rearrangement and a new growth cone. A cell body response to retrograde signaling induces profound changes in transcription and translocation in the neuronal cell body. The calcium wave elicits epigenetic changes in the soma enhancing histone acetylation required for initiation of transcriptional response. After the first wave of ion-flux-based signals there is a later, delayed, retrograde phase of signaling to the cell body by macromolecular signaling complexes on dynein motors. This is the main phase of retrograde signaling. There are a large variety of proteins identified but the most studied are JUN Amino-Terminal Kinase (JNK)-Interacting Protein (JIP) group of scaffold proteins and importins Figure 4.
Figure 4: Signalling events following peripheral axonal injury [15]. Increased calcium activates pro-regenerative pathways at the site of injury and in the soma.
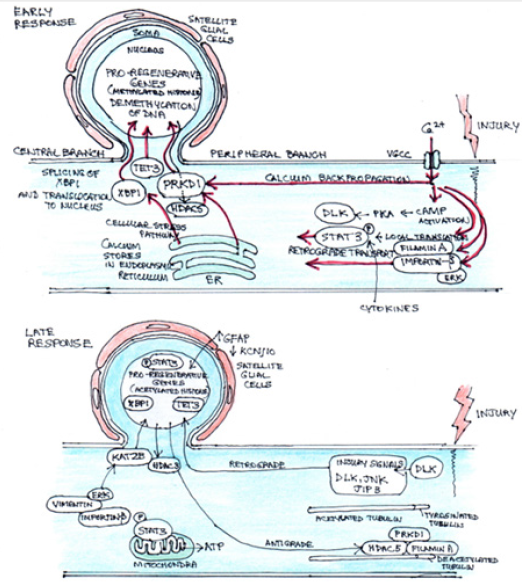
Early injury signaling events: Following injury, calcium rapidly flows into the damaged axon via Voltage-Gated Calcium Channels (VGCC) and is actively propagated to the soma. Calcium is also released from stores in the Endoplasmic Reticulum (ER). Release of stored calcium also triggers a cellular stress pathway resulting in splicing of X-Box-Binding Protein 1 (XBP1) and its translocation to the nucleus. Near the injury site calcium activates cAMP, which in turn activates PKA and Dual Leucine Zipper-Bearing Kinase (DLK), a chief mediator of later regenerative events. Also, at the site of injury, calcium facilitates translocation of STAT3, importin-β and filamin A. The association of ERK with importin allowing retrograde transport. Propagation of calcium to the soma promotes translocation of serine/threonine Protein Kinase D1 (PRKD1) to the nucleus, promoting Histone Deacetylase 5 (HDAC5) export. The calcium also facilitates expression of Methylcytosine Dioxygenase (TET3) promoting de-methylation of DNA. STAT3 is phosphorylated through action of cytokines and is retrograde transported to the nucleus to activate regenerative programs.
Late injury signaling events: DLK enables retrograde transport of injury signalling proteins to the nucleus: including JUN N-Terminal Kinase (JNK), JNK-Interacting Protein 3 (JIP3) and DLK itself, facilitated by an increase in tyrosinated tubulin near the injury site. Phosphorylated STAT3 can localize to the mitochondria to increase ATP synthesis, or to the nucleus to influence transcription of pro-regenerative genes. Retro-transported ERK causes histone acetyltransferase (KAT2B) to be transported to the nucleus. Meanwhile, HDAC3 and 5 are transported out of the nucleus, HDAC5 transported to the growth cone where it interacts with filamin A and PRKD1 to deacetylate microtubules. The changes in subcellular localization of these histone modifiers, in association with calciumdependent increase in TET3, causes transition from repressive DNA with histone methylation into permissive acetylation, allowing relaxation of DNA around pro-regenerative genes, which allows binding by transcription factors and the expression of the pro-regenerative genes. Satellite glial cells respond to injury by signalling with upregulation of glial fibrillatory acidic protein (GFAP) and down regulation of ATP-dependent inwardly rectifying potassium channel Kir4.1 (KCNJ10) but it is still unclear how the SGCs receive the injury message.
Discussion
I am a clinician with a special interest in melanoma pathogenesis. I initially investigated the peripheral nerve injury response that augmented melanoma malignancy, mediated, in part, by the glial Schwann cell. It became clear that there was also a significant response by the sensory ganglionic cell body to neuronal injury. The glial support to the ganglionic cell body is provided by the SGC, to which it maintains a close physical presence, controlling its physical and chemical environment. Until recently the role of the SGC has been given minimal recognition, understandably, because of the difficulty of studying these thin cells and isolating their function in vitro or in vivo. The glial support cells, Schwann cells and SGCs, as well as neuronal components of the peripheral nervous system are all derived from neural crest cells. These cells maintain a high degree of plasticity to allow for adaptation to environmental change and provide some regenerative potential at the periphery, particularly in relation to their function within the skin. They are immune active, so not only do they involve themselves but also encourage the influx of the systemic macrophages, through chemoattractive cytokine release. The macrophages also demonstrate plasticity in response. All these changes and information is reported back to the CNS [15].
This raises some other important questions. Is there an analgesic option for the management of chronic or neuropathic pain by manipulation of SGC communication to and from the peripheral ganglionic cell body? Can the more central neuronalglial communications be similarly influenced? And, is it possible for the translational and transcriptional regenerative response of the peripheral nervous system to be reproduced in the central nervous system’s glia and neurons to help repair damage from spinal injury or neuro-degenerative conditions?
References
- Koike T, Wakabayashi T, Mori T, Hirahara Y, Yamada H, et al. (2015) Sox2 promotes survival of satellite glial cells in vitro. Biochem Biophys 464(1): 269-274.
- Arora DK, Cosgrave AS, Howard MR, Bubb V, Quinn JP, et al. (2007) Evidence of postnatal neurogenesis in dorsal root ganglia: Role of nitric oxide and neuronal restrictive silencer transcription factor. J Molec Neurosci 32(2): 97-107.
- Li H, Say E, Zhou X (2007) Isolation and characterization of neural crest progenitors from adult dorsal root ganglia. Stem cells 25(8): 2053-2065.
- Takahashi M, Osumi N (2005) Identification of a novel type II classical cadherin: Rat cadherin 19 is expressed in the cranial ganglion and Schwann cell precursors during development. Dev Dyn 232(1): 200-208.
- George D, Ahrens P, Lambert S (2018) Satellite glial cells represent a population of developmentally arrested schwann cells. Glia 66(7): 1496-1506.
- Vetzen VM, Laman JD, Kleinjan A, Poot A, Albert DME, et al. (2009) Neuron-interacting satellite glial cells in human trigeminal ganglia have an APC phenotype. J Immunol 183(4): 2456-2461.
- Vit J, Ohara P, Bhargava A, Kelley K, Jasmin L (2008) Silencing the K4.1 potassium channel subunit in satellite glial cells of the rat trigeminal ganglion results in pain-like behaviour in the absence of nerve injury. J Neurosci 28(16): 4161-4171.
- Hanstein R, Hanani M, Scemes E, Spray DC (2016) Glial pannexin 1 contributes to tactile hypersensitivity in a mouse model of orofacial pain. Sci Rep 6: 38266.
- Belzer V, Hanani M (2019) Nitrous oxide as a messenger between neurons and satellite glial cells in dorsal root ganglia. Glia 67(7): 1296-1307.
- Watkins L, Malar S (2003) Glia: A novel drug discovery target for clinical pain. Rev Drug Nat Discov 2(12): 973-985.
- Yatziv S, Devor M (2019) Suppression of neuropathic pain by selective silencing of dorsal root ganglion ectopia using non-blocking concentrations of lidocaine. Pain 160(9): 2015-2114.
- Jin Y, Zhang P, Hao T, Ming LW, Di Guo M, et al. (2019) Connexin 43 contributes to temporomandibular joint inflammatory induced-hyper-nociception via sodium channel 1.7 in trigeminal ganglions. Neurosci Lett 707: 134301.
- Armselem M, Poilbout C, Ferracci G, Delmas P, Padilla F (2018) Membrane cholesterol depletion as a trigger of Nav 1.9 channel-mediated inflammatory pain. The EMBO J 37(8): e97349.
- Zigmond R (2012) Cytokines that promote nerve regeneration. Exp Neuro 238: 101-106.
- Mahar M, Cavalli V (2018) Intrinsic mechanisms of neuronal axon regeneration. Nature reviews 19: 323-337.
© 2021 David John Mackay Smith. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






