- Submissions

Full Text
Novel Research in Sciences
Designation of High Branched Polyurethane Composite Coating from Recycling of Pet/Epoxy for Applying as Corrosion Protection
Ossama M Abo Elenien1*, Olfat E Elazabawy1, Mohamed A Abbas1, Alaa I Eid2 and Salah M El Bahy3
1Petroleum Application Department, Egyptian Petroleum Research Institute (EPRI), Nasr City 11727, Cairo, Egypt
2Faculty of Chemistry, Kabardino-Balkarian State University (KBSU), Nalchik, Russia
3Department of Chemistry, Turabah University College, Taif University, P.O.Box 11099, Taif 21944, Saudi Arabia
*Corresponding author: Ossama M Abo Elenien, Petroleum Application Department, Egyptian Petroleum Research Institute (EPRI), Nasr City 11727, Cairo, Egypt
Submission: July 14, 2021;Published: August 27, 2021
.jpg)
Volume9 Issue2August, 2021
Abstract
In this study, wastes bottles of Polyethylene Glycol Terephthalate (PET) were recycled via glycolysis process using Pentaerethretol, to be reused as starting ingredients for new production polymer. It is shown that, the chemically recycled PET by esterifying the polyester with an excess of reactant such as Pentaerethretol (Per) in the attendance of zinc acetate as a developer at 190-220 OC for 8 hrs. The produced (PEGPEr) oligoester from PET waste glycolysis was introduced as a starting material in the manufacturing of High Branched Poly (hydroxy ethylene terephthalate pentaerythritol bis-phenol A) resin (HBPHETPErBPAT), its happened through the reaction of PET-GPEr oligoesters with bis phenol epoxy A at 200-220 oC for 5hrs, the composite product of which is suitable to produce a High Branched Polyurethane (HBPU) coating. A possibility of new application of HBPHETPErBPAT resin and Toluene Di-Isocyanate (TDI) as curing agent coatings are applied on the prepared surface of steel specimen. The physical, mechanical and chemical characteristics of the obtained high branched polyurethane films are there investigated. Also salt spray test and cathodic disbandment be present estimated of formed dry films on carbon steel specimens, the obtained HPPU coating films from this reason should be increasing the film strength and durability.
Keywords:PET waste; HBPHETPErBPAT composite resins; TDI; HBPU coating; Corrosion protection and cathodic disbondment
Introduction
Waste polyethylene terephthalate recycling processes are the greatest way to economically [1]. Price of virgin PET remainders a stable, novel and inexpensive technology for recycling industry by providing industry with relatively cheaper PET. The recycling of PET was initially in 1977 [2]. PET glycolysis remains reflected a trans-esterification reaction. The uses of metallic acetates as catalysts were investigated [3,4]. The widespread of polymer recycling and greatest fruitful are poly (ethylene terephthalate) (PET) waste recycling [5-8]. While the PET recovering does not assist by way of a fractional solution to the solid as raw industrial material. A chemical recycling is signs to the formation of the fresh materials, over and above of added subordinate value [7]. Only just the rising consideration ensures pragmatic trendy custom instead of the manufacture of specific produces as per painting resin, polymer concrete unsaturated polyester and polyurethane foams [9-13]. Owing to the widespread coatings in different fields as petroleum industries (pipelines, tanks, vessels and steel structure), shipbuilding and automotive businesses, which is interested as enterprises, expending in the conformation alternate to reduction material goods fundamentally [14] his polyols [15-19].
Some gums were ready from depolymerization product of the waste PET coat [6,17]. Current effort polyol kauri gum built on the recycled to polyol form. The goal is remained to establish lower molecular weight, which were interacted with bisphenol epoxy A to produced, having high branched ratio of OH groups HBPHETPErBPAT composite, HBPU coats to protect petroleum (pipelines, tanks, vessels and steel structure), treated many playing as sea atmospheres, workroom faster. Simultaneously, deterioration proposed in exploration.
Procedures
Materials
Waste PET bottles are collected and cut into small pieces 3mm2, cleaner by aquatic, then by acetone several time and dry at 100 °C for 8hrs. The pentaerytheritol (PEr), zinc acetate and solvents are obtained from (Aldrich Chemical Co.). Commercial of Epoxy base phenol A and toluene di-isocyanate were obtained from hunts man Co.
PET-GPEr resins synthesis
The reaction PET flak 250g with PEr 75g and zinc acetate 3g, as catalyst was passed out in three neck flasks, fortified with agitator, thermometer, nitrogen creek and reflux system without solvent. The reaction mixes were heated at 150-170 °C for 3hrs, raised to 190 °C and finally to 220 °C for 5hrs. Hotness dropped allowable chamber degree. Cleansing be present achieved done through warm liquid. After completing glycolysis, cool the yield to near room temperature, liquefy it with a sufficient amount of organic solvent, and shake vigorously with an equal volume of 5% NaCl solution to remove any unreacted PEr and residual catalyst. The organic amount was combined and washed away with water several times. Acid and alkali identified the PET-GPEr oligomer.
Preparation of HBPHETPErBPAT composite resins
From 200g of PET-GPEr oligoester and 300g of epoxy base phenol A, an HBPHETPErBPAT composite resin was developed. The reaction was carried out in a round bottom flask with a touch thermometer, nitrogen creek, and an automated stirrer device in place. For 5 hours, the reaction temperature was maintained at 110-120 °C. After the reaction was completed, the resins were dissolved in toluene, xylene, and methanol.
Preparation of HBPU coating from HBPHETPErBPAT composite resins
The TDI is used to make the HBPU coating (Toluene Diisocyanate). In a 250ml beaker, TDI was applied to prepared HBPHETPErBPAT Composite Resins at different ratios of 20, 25, 30, 35, and 40%, while stirring vigorously for around 20 minutes. Then, using an air sprayer, apply the prepared polyurethane to the steel panel’s preparation surface. The polyurethane coats obtained are PU1 (20 percent TDI), PU2 (25 percent TDI), and PU3 (30 percent TDI).
Measurements
Using a Mattson-Infinity series FTIR Bench Top 961, the infrared spectrum of polymer/KBr pellets was investigated. On a 270MHz spectrometer W-P-270 & Y Bruker, 1H NMR spectra of ready resins were detailed. Running the prepared compound in CDCl3 coordinated the 1HNMR study. GPC (Waters model 510) was used to determine the molecular weight, with THF (HPLC grade) as the eluent and Ultra styra gel 500; 100.
The HBPU coatings were put to the test
To evaluate the different properties of HBPU coatings, mild steel panels (15cml 1cm) are commonly used. The sheets’ other side is coated with free coal tar epoxy, which makes them corrosion resistant. The panels’ mechanical properties and longevity are estimated, as well as their mechanical properties (pull off test, pencil stiffness, impact, T-bend investigations). As defined in our paper, chemical conflicts (hot water, acid, alkali, and solvent resistance) were calculated using ASTM methods (15) After immersion in 10% H2SO4 and 5% NaOH aqueous solutions using distilled water, the acid and alkali resistances of coated panels were high. The experiment lasted 90 days at a temperature of 22 °C. The salt-spray resistance of the panels was calculated in accordance with ASTM B117. 37 degrees Celsius, 95% relative humidity, and 4.2 percent aqueous sodium chloride way out were the test conditions. The coated panels’ adhesion grade and optical inspection of blisters and cracks is calculated. The ratio of coating to resistance Cathode Disbandment (CD) was determined using ASTM standards.
Discussion and Results
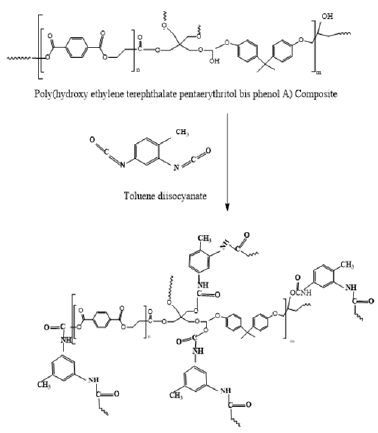
The use of PET waste suggests a lower charge spring for the creation of treatable resins. The aim of the current research was to create and describe an HBPU coating made from HBPHETPErBPAT Composite Resins, as well as to assess the mechanical and chemical resistance of the cured HPPU coating. The current study describes the reprocessing of PET waste through glycolysis with Pentaerethretol in the presence of zinc acetate as a catalyst to produce EPT-GPEr, which was then reacted with Epoxy base phenol A to produce HBPHETPErBPAT Composite Resins derivatives, as shown in Scheme (1), which were then reacted with toluene diisocyanate to form HPPU coating, as shown in Scheme (2).
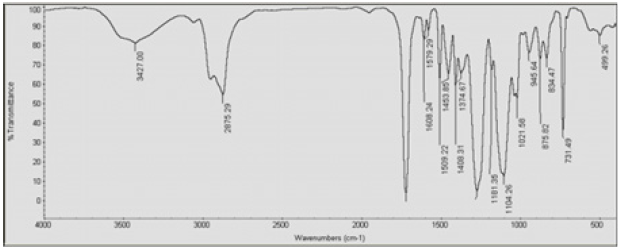
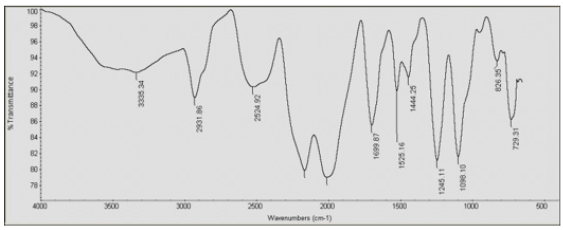
IR spectroscopy was used to validate the structures of HBPHETPErBPAT Composite Resins and HBPU. Figure 1 shows the IR spectra of these compounds (a,b). The characteristic bands in the IR spectra of HBPHETPErBPAT Composite Resins (Figure 1a) are found at 3427, 1408 Cm-1 (OH) groups, 2973 Cm-1 (CH) aromatics, 2875 Cm-1 (CH) aliphatic, 1700 Cm-1 (C=O in CH2 (C=O) group), 1608 Cm-1 (C=O) group, and 1608 Cm-1. The characteristic brood bands are observed in the IR spectra of HBPU (Figure 1b) at 3335 and 2524 Cm-1 (amide) pairs, 2973 Cm-1 (CH) aromatics, 2875 Cm-1 (CH) aliphatic, 1699 Cm-1 and 1530 Cm-1 (C= O, sym and C= O, asym in amides and ester) and at 1525Cm. the complete reaction (consumed) of HBPU causes the bands characteristic of OH groups to disappear. For the HBPHETPErBPAT Composite resins Figure 2 and Table 1, the GPC data of number and weight average molecular weights and polydispersity have been estimated as 2996, 8108, and 2.706g mol-1, respectively.
Figure 1: Preparation of high branched Poly (hydroxy ethylene terephthalate pentaerythritol bis pheonl A) Composite Resins from glycolized Poly (ethylene terephtalate) (PET) waste oligoester.
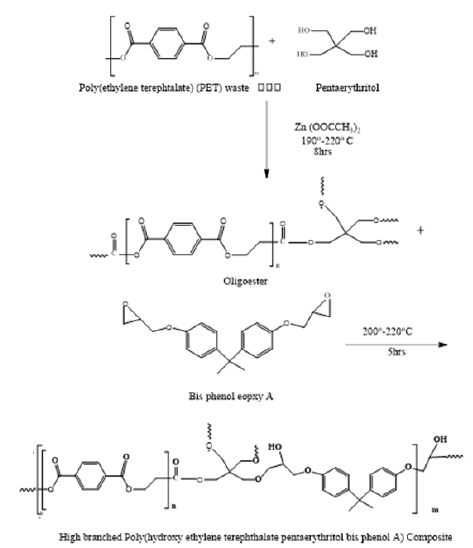
Table 1: Results data of GPC for HBPHETPErBPAT composite resins GPC sample results.

Figure 2:
1HNMR spectroscopy was used to investigate the structure of HBPHETPErBPAT Composite Resins, as shown in Figure 3. In this case, two peaks with the same number of protons are compared for their integration values. Integrations of CH2 protons close to the OH group of PEr were linked to integrations of CH2 protons attached PET in the current study. Peak integration is found to be 15.96 at =4.4ppm (assigned to one methylene protons of ethylene glycol of PET) and 7.76 at s=4.18ppm (assigned to the methylene group of the ethyl moiety of PEr). Per and bis phenol A are mostly found at chain ends, according to the data. HBPHETPErBPAT Composite Resins with high OHs and branching are suitable polyols for use in product systems.
Figure 3: High branched Polyurethane from Poly (hydroxy ethylene terephthalate pentaerythritol bis pheonl A) Composite resins of Poly (hydroxy ethylene terephthalate pentaerythritol bis pheonl A).
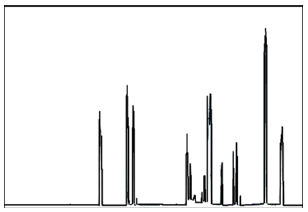
TGA and DSC data from HBPHETPErBPAT Resins showed relative thermal stability until 139 °C, where weight loss was only 5%, and DSC measurements reported Tg value as 105 °C, whereas at PU5, thermal stability reached 161 °C by weight loss of 5%, and DSC measurements recorded Tg value as 130 °C, indicating high thermal stability of organised HBPU from HBPHETPErBPAT. This means that incorporating HBPHETPErBPAT Composite Resins into HBPU coatings has a decomposition-stabilizing effect. In Figure, the DSC-TGA of PU5 was designated and signified (Figures 4a & 4b). Show to improve their appearance, longevity, scratch and corrosion resistance among the various resin materials used in polymeric applications. Diisocyanates and low molar mass diols form segmented block copolymers in universal PUs to form composed hard sectors. Polyurethane with different material properties results from the use of polyol Resins as pioneers with a close construction instead of branched polyether or polyester polyols [13].
Figure 4:FTIR spectra of (a) HBPHETPErBPAT Composite Resins and b) HBPU coating.
Mechanical properties of the dried HBPHETPErBPAT composite resins to HBPU coating
HBPHETPErBPAT Composite Resins can be integrated into an HBPU matrix as blends or added into the network as a cross linker, so the HBPHETPErBPAT Composite Resins were prepared in this study. HBPHETPErBPAT Composite Resins are cured with TDI in various ratios to form a Hyper-Branched Polyurethane Network (HBPU). The dried yields have excellent chemical resistance and physical strength. Salt spray and alkaline resistances are mildly difficult for them. The production of new HBPU resins has focused on two aspects of polyurethane resins: the development and modifications of newer polyurethane from PET waste glycolysis, which is a low-cost resource, and their applications in adhesives, paints, varnishes, building materials, and other advanced fields (Table 2).
Table 2: Thermal analysis (TGA & DSc) of HBPHETPErBPAT composite resins AND cured HBPU coating.
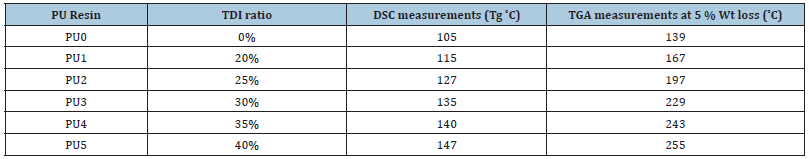
The aim of this study is to make HBPHETPErBPAT Composite Resins with toluene diisocyanate to make cured HBPU films with good durability and excellent physical, chemical, and mechanical properties, as determined by effect, adhesion, and hardness tests. The composite samples were sprayed on the steel panel preparation surface (using an air spray gun).
After 7 days at room temperature, the mechanical properties were evaluated. Table 3 contains the data for mechanical properties. These figures show that cured polyurethane PU5 with a TDI of 40% has superior adhesion properties to steel. This can be attributed to the dried film’s high crosslinking due to the increased number of OH groups in HBPHETPErBPAT Composite Resins, which meets the necessary amount with TDI. With impact and T-bend studies, PU5 produces the best performance. These characteristics indicate that the ratios of HBPHETPErBPAT Composite Resins to crosslinking agents have an emotional effect on the coatings’ mechanical properties. Because of these appearances, one might assume that high crosslink density networks have strong perfunctory properties. These PU arrangement properties can also be used to estimate the cure evaluation. The difference in hardness results from softer (minimum cross links) to harder (maximum cross links) coatings revealed this (maximum cross-link-density)
Table 3:Coating tests of polyurethane resin cured by TDI.
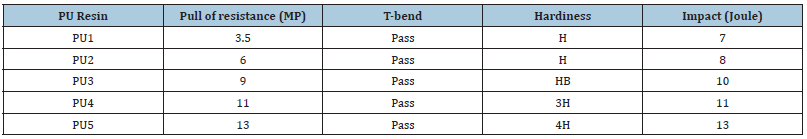
Chemical resistance of cured resins
In order to assess the coats’ stability, the covered plates were exposed to alkali, acid, solvent, and salt spray.
The failure of the test demonstrates that the coating films lose their adhesion to the panels, as well as cracking and flaking. There are several causes for coating disappointment. However, certain causes of disappointment are easily detectable, and efforts can be made to piece them together. Degradation kernels exist in architectural coverings based on autoxidizable binders. When the film is dry, the oxidation process continues. The suitability of modern exterior coating stability is dependent on the quality of the binder. The criteria will be flawless, as they will include both chemical defence and optimal mechanical assets. Coating device failure can be caused by one or more of these causes, or a combination of them. We will now address a wide variety of tests, and the results of these tests will be used to provide an estimate of the likely output of shaped films, which will aid in their stability. Solvent resistance can be tested for a variety of reasons. To determine the degree of cure of a cross-linked composition, tropical solvents such as ketones are often used; methyl isobutyl ketone or acetone are recommended. A rub test can be used to assess solvent resistance in addition to dipping analysis.
Acetone is used to determine the degree of drying of the current coating systems using both dipping and rub methods in this regard. The disorder and dissolution of the shaped films from the panels dictated whether or not the tests were passed. Solvent tolerance is usually measured by the polarity of cured network resins. Air, acetone, and other tropical solvents are resistant to non-polar polymers, whereas polymers with hydrogen bonding sites are most influenced by moisture humidity and polar solvents.
Furthermore, the crosslink concreteness and molecular weight of polymer networks are linked to their solvent resistance. The thermodynamic relationship between polymer network structures and solvent explains this. The ultimate structural factors in preventing a polymer from dissolving in a solvent are cross links. Although this does not completely remove the effects of polarity and hydrogen bonding, it does increase molecular weight to the size of an infinite network, which prevents individual polymer chains from dissolving in the solvent. The more cross linking there is, the less free volume there is, and segmental mobility in the polymer remains. As a result, the solvent particles are unable to join the network. The ratio of curing agent and functionality of resin hydroxides can be used to monitor the crosslink concreteness.
The chemical resistance data of polyurethane were reported in Table 3 in this regard. All PU films based on HBPHETPErBPAT Composite Resins were found to have upright solvent resistance. This can be attributed to an increase in crosslink density due to the addition of hydroxyl functional groups, which increases the crosslink of polyurethane by increasing the TDI to HBPHETPErBPAT ratio. The chemical resistivity of the device with a low TDI ratio (30%) to HBPHETPErBPAT Composite Resins has been observed to fail. This is due to a decrease in network crosslink density and an increase in acidic and alkaline solutions attacking the network’s hydroxyl groups, but PU5 has the highest chemical resistivity when the TDI is greater than 40%, and when the TDI is greater than 40%, the chemical resistivity decreases. The results of acid and alkali resistance tests on cured polyurethane show that these networks are highly resistant to alkali and acid aqueous solutions. The networks’ high crosslink concentration reduces their exposure to the environment.
HBPU coating corrosion resistance
Salt spray tests are perhaps the most common and contentious corrosion resistance tests. It is well known that sodium chloride can cause rapid corrosion of ferrous substrates, so knowing how well a specific structure protects the substrate surface from corrosion is useful knowledge. They are, however, well-established, and despite the issue of reproducibility, they are reasonably useful guides to presentation in the absence of longer-term decay data.
Because of the degree of acceleration of the degradation phase that they achieve and the uncertainty of the level of harm that is affected in some of the experiments, they are considered impractical by some staff. The continuous salt shower test is the first, and the intermittent salt shower test is the second. The continuous salt shower test is used to determine the effect of salts on the material, as measured by the quality of the shaped films. For all obtained polyurethane films based on HBPHETPErBPAT Composite Resins and TDI, the period times of the examinations are calculated and reported in Table 4. Figure 5 shows images of the coated panels of PU5 and PU1 after exposure to salt spray. Increased cross related concentration indicates high adhesion of coatings, according to the salt spray findings (Table 5).
Table 4: Chemical resistance tests of cured polyurethane resin.
Figure 5:GPC spectrum for HBPHETPErBPAT Resins.
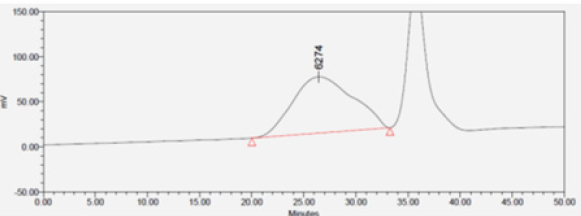
Table 5: Salt spray resistance of cured PU resins.
For both PU5 and PU1 coatings, coating performance improved with each exposure period. This can be attributed to coating properties and efficiency families. The adhesion of the coat to the substrate is the secret to successful coating in this detail. Under rusting does not cause cracking, flaking, scaling, or blistering. In polyurethane systems, some terminated hydroxyl groups increase the bond between the coat and the steel by forming a physicchemical bond between them. This phenomenon can be linked to the production of polyurethane groups by curing hydroxyl groups with TDI hardener. The excess of hydroxyl group also has a negative impact on the adhesion of the polyurethane coating to the metal surface. This is approved for the fully cure of PU5, which is a high interconnected network that increases their resistance to chemicals and salt solutions, and thus increases their corrosion resistance; however, in the case of PU1, the network is not completely cure enough, so the cross-linked film is weak, and if the TDI ratio to HBPHETPErBPAT is too high, the cross-linked film is weak. Resins for Composites HBPHETPErBPAT receives less attention. Composite resins on the film, which interfere with the healed film’s properties.
Cure HBPHETPErBPAT Composite polyurethane has excellent alkali, acid, and solvent resistance and provides a thick hard coating with strong adhesion in this current configuration. As a result of this evidence, it can be used in linings for petroleum equipment. The PU coating of PET derivatives, which has exceptional resistance to osmosis and electro endosmosis as compared to aliphatic [20], is responsible for the high salt spray dispute of cured HBPHETPErBPAT Composite Resins. Cathodic Protection (CP) with an impressed current is often used to protect buried steel from corrosion. The Cathodic Disbondment (CD) test is one of the most common failure modes for organic coatings on steel surfaces, and it can result in the metal substrate losing its corrosion safety.
Cathodic delamination, or the failure of the bond between the coat and the steel surface, is caused by cathodic action underneath the coat.
The CD data showed that the first radius before the test was 6mm, and after the final test was 7.3mm, the increasing radius was 1.3mm, resulting in a 21.667mm increase in area. As a result, HBPU is an excellent protective coating. The CD test was conducted for 48 hours at 65 degrees Celsius. The images of the cured HBPU coating were chosen to illustrate the CD test results depicted in Figure 6. According to the CD results, cathodic disbandment resistance was provided by well-adhered HBPU coatings on steel substrates. In general, conditions that allow oxidation of polyurethane-based coatings are needed for good bonding of polyurethane coatings to steel. It has been proposed that adding polar functional groups to polyurethane coatings increases their CD efficiency [21,22].
Figure 6:1HNMR Spectrum of HBPHETPErBPAT Composite Resins.
The formation of a dipole-dipole interaction between the polar groups of HBPU coatings and the metal oxide of the steel substrate was credited with this. Because of the risk of deprivation of the polar moieties in the presence of alkali produced during CD reactions, there is a slight reduction in CD resistance for higher loadings of polar groups. As a result, this finding contradicts our hypothesis that a larger number of functionalized ester groups improve CD results. Essentially, these types of alkyd materials may be used as exterior coverings for the bottoms of boot-topping and decks, as well as gasoline pipeline, tanker, and transports of general chemical tankers (Figures 7-9).
Figure 7:DSC-TGA thermal characteristics of a) HBPHETPErBPAT Composite Resins, and b) for cured HBPU coating PU5.
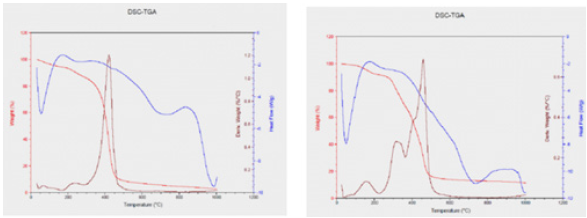
Figure 8:a) salt spray resistance of PU5 befor b) salt spray resistance of PU5 at 1000 hours.
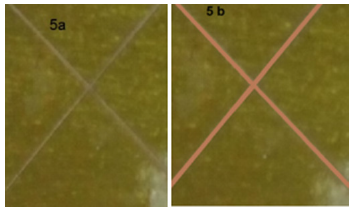
Figure 9:Cathodic disbondment of HBPU coating before and after test in 5% NaCl at 65 0C and 48hrs.

Conclusion
The corrosion resistance of carbon steel was tested using HBPUbased protective coatings. All of the coatings have been identified using standard techniques such as spectroscopy and viscosity measurements. The following are the most critical decisions that can be made as a result of the findings of this study:
a) All cured HBPHETPErBPAT Composite Resins have greater adhesion properties with steel than other cured resins, according to the data published on adhesions (pull-off). This can be attributed to the formation of a uniform, homogeneous network of resins, as well as the existence of terminal hydroxyl groups, which improved steel adhesion.
b) According to the results, the HBPU has good mechanical properties. These actions indicate that crosslink concentration ratios have an effect on the mechanical properties of coatings.
c) As polyurethane coatings, the cured HBPHETPErBPAT Composite Resins have excellent chemical resistance, according to the results. Alkaline and acidic tolerance can be attributed to high cross-link density in networks, which reduces their interaction with the environment.
d) The results of salt spray and cathodic disbondment showed that HBPU coatings have a strong bond to the steel surface and produce good results.
Acknowledgement
The authors gratefully acknowledge financial support from Taif University Researchers Supporting Project number (TURSP-2020/135), Taif University, Taif, Saudi Arabia.
References
- Throne JL (1987) Effect of recycle on properties and profits: Algorithms. Adv Polym Technol 7(4): 346-360.
- Yoda N (1988) Corporate management in the age of global environment awareness-a case study of pet bottle recycling issues in Japan. Science and Technology of Polymers and Advanced Materials, USA.
- Chujo Y, Kobayashi H, Yamashita Y (1989) J Polym Sci Chem Ed 27: 2007-2014.
- Nikles DE, Farahat MS (2005) New motivation for the depolymerization products derived from Poly(Ethylene Terephthalate) (PET) Waste: a Review. Macromol Mater Eng 290(1): 13-30.
- Coelho TM, Castro R, Gobbo JA (2011) PET containers in Brazil: Opportunities and challenges of a logistics model for post-consumer waste recycling. Jr Conserv Recycl 55(3): 291-299.
- Torlakoglu A, Guclu G (2009) Alkyd-amino resins based on waste PET for coating applications. Waste Management 29(1): 350-354.
- Barboza ES, Lopez DR, Amico SC, Ferreira CA (2009) Determination of a recyclability index for the PET glycolysis. Conserv Recycl 53(3): 122-128.
- Abo-Elenien OM (2006) Synthesizes and performance of poly (Ethylene Glycol Terphthalate Dimethyl Siloxane) compound as protective material for carbon steel alloy of petroleum pipelines. Chemical Technology 1(2-4): 63-71.
- Abdel-Azim AA, Atta AM (1998) Recycled flexible resins in concrete. Polym J 29(1): 21-24.
- Atta AM (2003) Epoxy Resin Based on Poly (ethylene terephthalate) Waste: Synthesis and Characterization. Prog in Rubber, plastics and Recycl Technol 19(1): 17.
- Abdel Azim AA, Atta AM, El-Ghazawy RA (2006) Synthesis of rigid polyurethane foams from recycled poly(ethylene Terephthalate) Waste. Cellular Polym 25(1): 35-48.
- Atta AM, Abdel-Raouf ME, Elsaeed SM, Abdel-Azim AA (2006) Curable resins based on recycled poly(ethylene terephthalate) for coating applications. Prog Org Coat 55(1): 50-59.
- Atta AM, El-Kafrawy AF, Aly MH, Abdel-Azim AA (2007) New epoxy resins based on recycled poly (ethylene terephthalate) as organic coatings. Prog Org Coat 58(1): 13-22.
- Abo-Elenien OM, Abu-Alainin HM (2006) Synthesizes and performance of poly (ethylene glycol terphthalate dimethyl siloxane) compound as protective material for carbon steel alloy of petroleum pipelines. Chemical Technology an Indian Journal 1(2-4): 63-71.
- Patton TC (2002) Alkyd resin technology. John Wiley and Sons, Stuart, New York, USA, pp. 43-98.
- Karayannidis GP, Achilias DS, Sideridou ID, Bikiaris DN (2005) Alkyd resins derived from glycolized waste poly (ethylene terephthalate). European Polym J 41(2): 201-210.
- Guclu G, Orbay M (2009) Alkyd resins synthesized from postconsumer PET bottles. Prog Org Coat 65(3): 362-365.
- Dullius J, Ruecker C, Oliveira V, Ligabue R, Einloft S (2006) Chemical recycling of post-consumer PET: Alkyd resins synthesis. Prog Org Coat 57(2): 123-127.
- Akintayo CO, Adebowale KO (2004) Synthesis, characterization and evaluation of chlorinated Albizia benth medium oil alkyds. Prog Org Coat 50(2): 138-143.
- Meldrum DA, Lin CT (1993) J Coat Technol. 65(818): 47.
- Roy D, Simon GP, Forsyth M (2001) Blends of maleic-anhydride-grafted polyethylene with polyethylene for improved cathodic disbondment performance. Polym Int 50(10): 1115-1123.
- Kinloch AJ, Korenberg CF, Tan KT, Watts JF (2007) Crack growth in structural adhesive joints in aqueous environments. Mater Sci 42: 6353-6370.
© 2021 Imelda Orozco Mares. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






