- Submissions

Full Text
Novel Research in Sciences
Mammary Analogue Secretory Carcinoma of Parotid Gland: A Rare Entity
Ihtisham Khan1, Erum Ataullah2, Maria Khattak3* and Zubair Durrani4
Department of Oral and Maxillofacial, Pakistan
*Corresponding author: Maria Nizam, Trainee Dental Surgeon, Department of Oral & Maxillofacial, Pakistan
Submission: June 10, 2021;Published: July 02, 2021
.jpg)
Volume8 Issue3July, 2021
Case Presentation
A 27-year-old male presented to the oral and maxillofacial department of Rehman Medical Institute with a painless, firm swelling in the left parotid region present for the last two years. On examination, it was a 3x3 cm, non-tender lump. The facial nerve was intact with no regional lymphadenopathy. MRI was performed that showed a 2.7x2.5cm, well-defined mass in the superficial lobe of the left parotid gland. It was predominantly iso-intense on T1 with iso and hypointense components on T2W1. Post-contrast images showed homogenous enhancement in the lesion. No enlarged cervical lymph nodes were identified. FNA was performed, which showed numerous sheets of sebaceous type cells containing vacuolated cytoplasm in a background of cheesy type material. The nucleoli of the cells were variable in size with regular contours and uniform chromatin distribution. Definite atypia was not noted. A differential diagnosis was given as either sebaceous gland cyst or sebaceous gland adenoma. After a discussion in a multidisciplinary meeting, the decision was made to remove the lump. Partial superficial Parotidectomy was performed while pre- serving the facial nerve. His postoperative recovery was unremarkable. The histopathological examination showed salivary gland tissue infiltrated by a malignant tumor with cystic and tubular architecture consisting of intermediate-sized cells with amphophilic vacuolated cytoplasm. Intraluminal colloid-like material with a bubbly appearance was noted. Perineural invasion was also present. Immunohistochemistry showed tumor cells positive for S100 and negative for CD117 & P63. A diagnosis of Mammary analogue secretory carcinoma was made. The resection margin was less than 1mm away from the normal tissue. Histopathology results were discussed in a multidisciplinary meeting, and a decision was made against adjuvant radiotherapy. The patient is kept on closed follow-up and has not shown any recurrence after one year.
Discussion
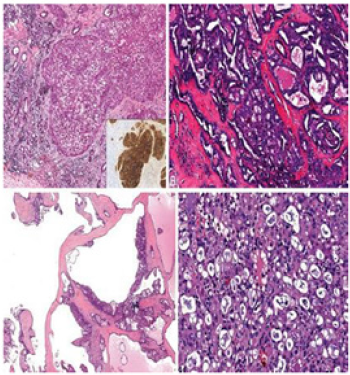
Mammary Analogue Secretory Carcinoma (MASC) is a newly described entity first described by Skalova et al. [1] in 2010. Characterized by morphologic and immunohistochemical features that strongly. Resemble Secretory Carcinoma (SC) of the breast, it originates in salivary tissue [1]. Occurring more commonly in males, with a wide age range from 13-77 years. Most patients present with a slow-growing mass with pain and facial paralysis reported in some cases [2-4]. Effecting most commonly the parotid gland (70% cases), it can also affect others [4-7]. The gross appearance of MASC generally shows a well-circumscribed, noncapsulated tumor with a rubbery consistency. Cut surfaces are grey, white to brown with the variable cystic component [3]. Histologically, MASC is predominantly an extra ductal neoplasm, typically showing at least some infiltration. Architecturally, MASC may show solid, microcystic, tubular, papillocystic, and cribriform patterns in varying proportions. Unicystic and multicystic examples of MASC (Figure 1) may occur, and these are typically no infiltrative and may be associated with hemorrhage and cholesterol clefts.8 Focal intraductal involvement by MASC may occur (Figures 2 & 3). Cytologically, MASC nuclei are small to medium-sized, oval to round, containing pale chromatin and an occasional single small nucleolus. Characteristically, the cytoplasm is abundant and eosinophilic to pink and bubbly. Microcystic and tubular spaces are often filled with eosinophilic colloid-like or frothy secretions [8]. Intracytoplasmic, mucicarmine-positive mucin may be found. Importantly, cytoplasmic zymogen granules are absent [3]. Mitotic figures are rare, and necrosis is typically absent. High-grade MASC examples have been reported and are characterized by a more reliable and trabecular architecture with necrosis, diminished secretions, and larger cells with more prominent nucleoli and atypia [2]. Various cytokeratins exhibit positivity for MASC like CK7, CK8, CK18, CK19, AE1/AE3, CAM 5.2. S100 and STAT5 a show strong & diffuse immunostaining. Gross cystic disease fluid protein (GCDFP-15) is also positive in the majority of cases [3]. Tumor cells and eosinophilic material will stain for mammaglobin. With appropriate morphology, the positivity of both S100 and mammaglobin are strong indicators of SC. However, these two stains alone are not entirely specific. Eosinophilic secretory material stains for PAS/PASD and Alcian blue. Myoepithelial markers: p63, calponin, CK14, SMA, and CK56 negatively stain for SC. Other immunomarkers like DOG1 and ER, PR also exhibit non-reactivity for SC [3]. While histopathological features of MASC overlap with those of other salivary gland tumors, such as acinic cell carcinoma (AciCC), adenocarcinoma, and lowgrade mucoepidermoid carcinoma, diagnosis of MASC is based on t (12;15) p (13; q25) translocation and positive immunochemical studies for STAT5 mammaglobin and S100 protein [7]. ACC is differentiated from MASC by the presence of large, serous, acinar cells with cytoplasmic PAS-positive zymogen like granules. Additionally, S100 protein is absent in ACC while strongly positive in MASC [9]. The definitive treatment for MASC is still not defined due to a lack of proper evidence. Local Excision may be performed. Adjuvant radiotherapy may be considered for positive surgical margin cases and high-grade tumors. Chiosea et al. [9] reported lymph node metastasis rates of 17.6% (6 of 34) in MASC, which may indicate a role for elective neck dissection in aggressive cases. Furthermore, the role of ETV6- NTRK3 inhibitors therapy is under investigation for prevention [10].
Figure 1:

Figure 2:
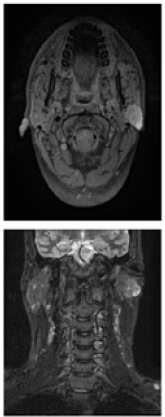
Figure 3:A. Solid pattern. B. Tubular & cribriform pattern. C. Macrocystic pattern. D. SC show low-grade carcinoma cells with abundant pink bubbly cytoplasm and frothy secretions [10].
References
- Skálová A, Michal M, Vanecek T, Sima R, Kinkor Z, et al. (2010) Mammary analogue secretory carcinoma of salivary glands, containing the ETV6-NTRK3 fusion gene: A hitherto undescribed salivary gland tumor entity. Am J Surg Pathol 34(5): 599-608.
- Connor A, Perez OB, Shago M, Skálová A, Weinreb I (2012) Mammary analog secretory carcinoma of salivary gland origin with the ETV6 gene rearrangement by FISH: expanded morphologic and immune histo-chemical spectrum of a recently described entity. Am J Surg Pathol 36(1): 27-34.
- Skalova (2013) Mammary analogue secretory carcinoma of salivary gland origin: an update and expanded morpho- logic and immunohistochemical spectrum of recently described entity. Head and neck pathology 7(1): 30-36.
- Sethi R, Kozin E, Remenschneider A (2014) Mammary analogue secretory carcinoma: update on a new diagnosis of salivary gland malignancy. Laryngoscope 124(1): 188-195.
- Majewska H, Skálová A, Stodulski D, Klimková A, Steiner P, et al. (2015) Mammary analogue secretory carcinoma of salivary glands: a new entity associated with ETV6 gene rearrangement. Virchows Arch 466(3): 245-254.
- Balanzá R, Arrangoiz R, Cordera F, Muñoz M, Luque DE, et al. (2015) Mammary analog secretory carcinoma of the parotid gland: A case report and literature review. Int J Surg Case Rep 16: 187-191.
- Bishop JA, Yonescu R, Batista DA, Westra WH, Ali SZ (2013) Cytopathologic features of mammary analogue secretory carcinoma. Cancer cytopathology 121(5): 228-233.
- Todd M, Stevens, Parekh V (2016) Mammary analogue secretory carcinoma. Arch Pathol Lab Med 140(9): 997-1001.
- Chiosea SI, Griffith C, Assaad A, Seethala RR (2012) Clinicopathological characterization of mammary analogue secretory carcinoma of salivary glands. Histopathology 61(3): 387-394.
- Skálová A, Vanecek T, Majewska H (2014) Mammary analogue secretory carcinoma of salivary glands with high-grade transformation report of 3 cases with the ETV6-NTRK3 gene fusion and analysis of TP53, beta-catenin, EGFR, and CCND1 genes. Am J Surg Pathol 38(1): 23-33.
© 2021 Maria Khattak. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






