- Submissions

Full Text
Novel Research in Sciences
Lipidic Classes Involved in Diabetes Mellitus, Review
Bourkoula A, Konsta E, Papadopoulou A and Trapali M*
Laboratory of Chemistry, Biochemistry, Cosmetic Science, University of West Attica, Greece
*Corresponding author: Maria Trapali, Laboratory of Chemistry, Biochemistry, Cosmetic Science, University of West Attica, Greece
Submission: June 23, 2021;Published: June 29, 2021
.jpg)
Volume8 Issue2June, 2021
Abstract
Diabetes mellitus is a metabolic disorder of multiple etiology, characterized by an increase in blood sugar concentration (hyperglycemia) and impaired glucose metabolism either as a result of decreased insulin secretion or due to decreased cells sensitivity to insulin. It is classified into two broad categories that do not concern the type of treatment or age but the etiology: Type 1 diabetes (autoimmune, idiopathic) is a disorder in which pancreas does not produce enough insulin. Type 2 diabetes mellitus (disorders of insulin secretion or action) is characterized by a disturbance in the action of insulin (insulin resistance) although the pancreas may produce increased amounts of insulin. Dyslipidemia is one of the main cardiovascular risk factors in diabetic patients. These patients show a specific dyslipidemic profile which is characterized by elevated triglyceride levels, normal or mildly elevated Low-Density Lipoprotein (LDL-C) cholesterol levels and low High-Density Lipoprotein (HDL-C) cholesterol levels. This review presents the main classes of lipids involved in the pathogenesis of Diabetes mellitus, which are triglycerides, phospholipids, and steroids.
Keywords: Diabetes mellitus; Dyslipidemia; Triglycerides; Phospholipids; Steroids
Lipidic Classes Involved in Diabetes Mellitus
Triglycerides
Triglycerides are the most abundant lipids in human adipose tissue and a major component of Very-Low-Density Lipoprotein (VLDL). According to National Cholesterol Education Program (NCEP) guidelines the desirable triglyceride level is below 150mg/dL [1]. According to the American Diabetes Association (ADA) the most common pattern of dyslipidemia in patients with type 2 diabetes is elevated triglyceride levels and decreased HDL cholesterol levels [2]. The condition in which triglyceride levels are elevated is called hypertriglyceridemia and can be classified into mild to moderate (triglycerides between 150- 499mg/dL) and severe hypertriglyceridemia (triglycerides ≥500mg/dL) [3]. Triglyceride values over 1,000mg/dL are accompanied by a serious risk of acute pancreatitis. In a retrospective cohort-based study with 1,586 patients with diabetes type 2 the prevalence of pancreatitis was 3.7% [4]. Several clinical studies [5-10] have demonstrated that elevated blood triglyceride levels increase the risk of Type 2 Diabetes (T2D). It is well recognized that diabetes is a risk factor for cardiovascular disease and has been associated with 2‐ to 4‐fold higher mortality [11] which is mainly due to atherogenic dyslipidemias characterized, among other factors, by elevated levels of triglycerides [12].
Recent randomization studies have demonstrated that hypertriglyceridemia is a causative agent in atherosclerosis [13,14]. The main step in order to reduce the risk of cardiovascular diseases related with diabetes is the detection and treatment of dyslipidemia which include the reduction of triglyceride levels. A study showed significant hypertriglyceridemia in all diabetic patients irrespective of the duration of diabetes and a significant negative correlation between triglyceride levels and heart rate variability whose reduction is related to increased mortality risk [12]. Hypertriglyceridemia has been characterized as an independent risk factor for diabetes type 2 incidence even after the control of Body Mass Index (BMI), hypertension and all other conventional risk factors [8,15,16]. The triglyceride levels and the triglyceride–HDL cholesterol ratio are important markers for identifying overweight persons who are insulin resistant, and they are in high risk for cardiovascular disease as much as ten years before diagnosis of T2D [17]. Zhao et al. [10] studied the association between triglyceride and diabetes type 2 among healthy middleaged and older adults in a prospective study with 8-year follow-ups in two cohorts. Their results showed that there was a significant linear association between triglyceride levels and the incidence of diabetes type 2. It is worth noticing that this association was independent of other factors such as age, sex, BMI and hypertension. A retrospective longitudinal study [5] which evaluated the risk of diabetes and prediabetes in the presence of a wide spectrum of triglyceride levels was carried out employing data from a screening center between the years 2000 and 2012. None of the study participants had evidence of diabetes while they had fasting plasma triglyceride level of 24-1130mg/dL at their initial visit. The results showed that every 10mg/dL increase in triglyceride levels increased the risk of diabetes by 4%. It is impressive that this association is valid even when the sustained increments in serum triglycerides level remained within the accepted normal range. Dotevall et al. [8] demonstrated that for middle-aged women without prior diabetes or cardiovascular disease, even very slightly elevated triglycerides levels resulted in significantly increased risk of developing diabetes regardless of age, BMI, blood pressure and physical activity. A very large population multicenter cross-sectional study [18] conducted between 2011 to 2013 in patients with type 2 diabetes showed that elevated triglyceride levels was the strongest factor among those associated with inadequate glycemic control in patients treated with sufficient dose of insulin and/or oral antidiabetic drugs. Due to the significant increase in prevalence of type 2 diabetes which is expected in developing countries between 2010 and 2030 [19], it is very important to identify individuals who have higher risks for diabetes in order to ensure early prevention and treatment.
Phospholipids
Phospholipids are a class a of lipids which represent the major component of the bilayer of the cell membrane and their structure consists of two major parts [20,21]. Two hydrophobic fatty acids “tails” and a hydrophilic “head” comprise a phosphate group. Phospholipids may also be divided into two groups through differences in their backbones, the one group, the glycerol-based phospholipids (glycerophospholipids) consists of phosphate groups which are modified with simple organic molecules such as choline (to form phosphatidylcholine), ethanolamine (to form phosphatidylethanolamine), inositol (to form phosphatidylinositol), serine (to form phosphatidylserine) or two phosphatidic acid moieties connected with a glycerol backbone in the centre to form cardiolipin. The other group contains the backbone of a sphingosine base, the sphingolipids [22]. Only sphingomyelin, which contains a phosphate group, belongs to this group of phospholipids. In mammalian cells, de novo synthesis of glycerophospholipids requires the acquisition of diacylglycerol units obtained through either diacylglycerol or Cytidine Diphosphate‐Diacylglycerol (CDPDAG) synthesized from phosphatidic acid [23,24].
Insulin resistance is the most common pathological factor accompanied by obesity which can be observed in patients suffering from diabetes type 2. The presence of insulin resistance in insulin-sensitive target tissues results in abnormalities such as hyperglycaemia, hyperinsulinemia, and hypertriglyceridemia, common features of the metabolic syndrome [25,26]. For decades, high triacylglycerol and cholesterol levels were considered to be the cause of these metabolic abnormalities, although recent studies have proposed that in addition to the raised triacylglycerol and cholesterol levels, phospholipid alterations could possibly play a role in metabolic disorders [27-30]. Decreased insulin sensitivity associated with decreased concentration of Polyunsaturated Fatty Acids (PUFA) in skeletal muscle phospholipids has been observed in clinical studies, which implies that changes in the fatty acid composition of the cell membranes could possibly affect the action of insulin [31]. A study conducted in older adults showed a positive relationship between the proportion of palmitic acid (C16:0) in the skeletal muscle phospholipids and the insulin sensitivity index [32]. This association was newly confirmed but it was also observed that the fatty acid composition of phosphatidylcholine and not phosphatidylethanolamine from skeletal muscle membranes was of importance in this association [33]. In that study, healthy patients were treated with nicotinic acid, an agent known to induce insulin resistance in humans. Treatment with nicotinic acid was associated with a 25% increase in the half‐maximal insulin concentration and decreased peripheral insulin sensitivity, and this was accompanied by significant increase in the phosphatidylcholine (C16:0), decrease phosphatidylcholine (C18:0) and long‐chain n‐3 fatty acid and PUFA. Recently, a clinical study in elderly participants also showed an association between decreased odds of abnormal homoeostasis model assessment‐insulin resistance (HOMA‐IR) and some phosphatidylcholine species (C32:0, C32:1, C32:2, C34:1, C34:2, C34:3, C36:2, C36:3, C40:5, C40:6, C42:3, C42:4 and C42:5) [34]. Furthermore, changes in the total content of phosphatidylcholine or phosphatidylethanolamine are also associated with insulin insensitivity. A clinical study conducted in lean control and overweight but non‐diabetic patients showed that baseline fasting plasma insulin and HOMA‐IR were positively correlated with erythrocyte membrane phosphatidylethanolamine and phosphatidylcholine content in the whole population [28]. Glycerolphospholipids, such as cardiolipin and phosphatidylinositol derivatives, have been shown to be associated with insulin sensitivity in clinical studies [35,36]. Cardiolipin content was increased by daily moderate‐intensity exercise in type 2 diabetic patients, and this was accompanied by improved insulin sensitivity [36]. Interestingly, obese patients with weight loss through gastric bypass surgery showed changes in only specific fatty acid species of cardiolipin after exercise without significant changes in total cardiolipin content [37]. Although the association between phospholipids and insulin sensitivity has been shown through clinical studies, it is not yet clarified whether changes in phospholipids are the cause or the consequence of insulin resistance.
Platelet activating factor: The Platelet Activating Factor (Platelet Activating Factor, PAF) is a lipoid which belongs to the class of phospholipids. Its name is: 1-O-alkyl-2-acetyl-sn-glycerol- 3-phospho-choline and Figure 1 [38]. PAF biosynthesis occurs through remodeling and de novo pathway. De novo pathway is believed to be responsible for PAF continuous production in blood and various tissues. Central enzyme in this process is alkyl acetylglycerol phosphocholino transferase (PAFCPT). Remodeling pathway is believed to be responsible for PAF production in inflammatory situations. Central enzyme is lyso- PAF: acetyl-CoA acetyltransferase (lyso-PAF-AT). Regarding PAF catabolism, the most important enzyme involved is PAF-acetylhydrolase (PAF-AH) which hydrolyzes short chain acyl groups from sn-2 position of PAF and forms lyso-PAF. PAF through its connection to one wellmarked receptor starts proliferation actions in pre-inflammatory cells involved in pathology of most chronic diseases, including cardiovascular and renal diseases, diabetes, central nervous system CNS diseases and cancer.
Figure 1: PAF structure.
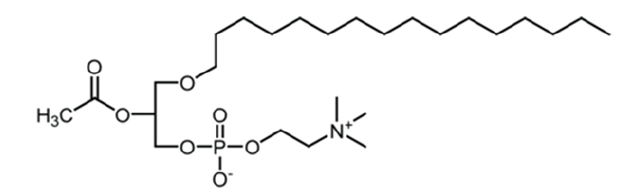
Inflammatory processes contribute significantly to the initiation, progression and rupture of lipid-rich atherosclerotic plaques. Atherogenic lipoproteins, including VLDL, VLDL remnants, IDL, LDL and Lp(a) are enhanced in atherogenic dyslipidemias such as hypercholesterolemia (Type IIA), mixed hyperlipidemia (Type IIB), and the dyslipidemia of Type II diabetes. The main points of atherogenesis mechanism due to PAF involvement is increase in blood levels, decreased levels of antioxidants molecules and PAFAH activity [39]. PAF like derivatives and oxidized phospholipids OxPLs, have been identified in atherosclerotic plaque. PAF-like molecules are formed when cell membrane is oxidized, resulting in compounds with smaller peroxidized residues on the second carbon which mimic PAF action. PAF-AH decomposes proinflammatory OxPLs and has a main role in lysophosphatidylcholine (lyso-PC) and oxidized fatty acids formation, many of which have inflammatory properties [39-41]. PAF like molecules and other vasoactive metabolites such as arachidonic acid, histamine, cytokines, chemokines and proteolytic enzymes can be released from mast cells during atherosclerotic plaque formation and cause hypercholesterolemia and hyperglycemia [42]. PAF role as an inflammatory mediator in pathogenesis of diabetes has been also studied. PAF levels are statistically increased in serum of diabetic patients while PAF levels in pancreas homogenates of three different groups of animals: well-fed, STZ-induced diabetic and fasted rats are elevated in diabetic animals [43,44]. PAF levels are also increased in plasma of pregnant women affected by Gestational Diabetes Mellitus (GDM), connecting inflammation, oxidative stress, and metabolic results [45] while PAF-AH shows a higher activity in patients with type 1 diabetes, which may be related to the higher risk of developing cardiovascular diseases observed in these patients [46].
Steroids
From the end of the last century, western societies have observed a notable increase of metabolic disorders including dyslipidemia, insulin resistance, hypertension, overweight and obesity [47], that will inevitably result in unprecedentedly high incidences of type 2 diabetes and cardiovascular disease worldwide [19,48]. For this reason, medical and scientific community has realized numerous studies on metabolic disorders focusing on steroid hormones, due to their fundamental role in the etiology and pathogenesis of metabolic disorders including obesity, diabetes and insulin resistance [49]. It is well known that glucocorticoids are extensively used in medicine either in short-term acute steroid therapy (exacerbation of chronic obstructive pulmonary disease, acute gout, chemotherapy protocols, bacterial meningitis and in pregnant women for fetal lung maturation, etc.) or in chronicterm steroid therapy in several diseases (pulmonary diseases, autoimmune conditions, neurologic diseases, inflammatory bowel diseases) as well as in modulating the immune system following solid organ transplantation. Although glucocorticoids are widely known in all medical specialties for their anti-inflammatory and immunosuppressive properties, they have various common metabolic side effects including hypertension, osteoporosis and diabetes. Specifically, for over 50 years, Steroid-Induced Diabetes Mellitus (SIDM) has been recognized as a complication of glucocorticoid use due to various reasons (impairment of multiple pathways including beta cell dysfunction, insulin resistance in other tissue, glucocorticoids effect on glyceroneogenesis in liver and adipose tissue). Diagnosing impaired fasting glucose or impaired glucose tolerance prior to the initiation of chronic glucocorticoids will better identify those who would benefit from steroid-sparing treatment, or if this is not an option, blood glucose monitoring while starting therapy. Further investigation into the precise mechanism of steroid-induced insulin resistance as well as exploring dose titration of insulin in patients on glucocorticoids possibly utilizing technology like continuous glucose monitoring system will provide insight into future diabetes prevention efforts and targeted therapies. It should be noted that as with all types of diabetes, initial steps to improve glycemic control include lifestyle modification (exercise and dietary counselling) to provide options that can perhaps lessen post-prandial hyperglycemia, but the mainstay of treatment is insulin therapy coincident with meals [50].
In addition, peripheral glucocorticoid metabolism through the promising avenue of selective 11_- hydroxysteroid dehydrogenase type 1 (11_-HSD1) inhibitors [51] as well as dysregulation of corticosteroid hormone production affect all metabolic diseases including obesity and insulin resistance. Whilst glucocorticoids have a potent impact upon metabolic phenotype, sex steroids (estrogens, estradiol) and physical activity play an equally important role in metabolic homeostasis [52-54].
Conclusion
it should be noted the potential risk of hyperglycemia in patients with diabetes following intra-articular steroid injections, that are widely used for the symptomatic control of degenerative and inflammatory joint arthritis. The benefits of steroids are due to their anti-inflammatory effects, as it has been already indicated. Locally injected steroids have been shown to be absorbed into the systemic circulation, affect glucose metabolism and can cause abnormal blood glucose levels in patients with diabetes. For this reason, patients with diabetes following intra-articular steroid injections need regular monitoring of blood glucose levels after injection for up to a week after injection, while they should seek medical advice if safe thresholds are breached [55].
Author Contributions
Conceptualization, MT; investigation, AB, EK and AP; writingoriginal draft preparation, AB, EK, DZL and AP; writing-review and editing, DZL; supervision, MT; project administration, MT. All authors have read and agreed to the published version of the manuscript.
References
- Miller M, Stone N, Ballantyne C, Bittner V, Criqui M, et al. (2011) Triglycerides and cardiovascular disease. A scientific statement from the American Heart Association. Circulation 123(20): 2292-2333.
- Haffner SM (2003) Dyslipidemia management in adults with diabetes. Diabetes Care 27: S68-S71.
- Jialal I, Amess W, Kaur M (2010) Management of hypertriglyceridemia in the diabetic patient. Current Diabetes Reports 10(4): 316-320.
- Albai O, Roman D, Frandes M (2017) Hypertriglyceridemia, an important and independent risk factor for acute pancreatitis in patients with type 2 diabetes mellitus. Therapeutics and Clinical Risk Management 13: 515-522.
- Beshara A, Cohen E, Goldberg E, Lilos P, Garty M, et al. (2016) Triglyceride levels and risk of type 2 diabetes mellitus: A longitudinal large study. Journal of Investigative Medicine 64(2): 383-387.
- Wilson P, Meigs JB, Sullivan L, Fox CS, Nathan DM, et al. (2007) Prediction of incident diabetes mellitus in middle-aged adults. The Framingham Offspring Study. Archives of Internal Medicine 167(10): 1068-1074.
- Schmidt M, Duncan B, Bang H, Pankow J, Ballantyne C, et al. (2005) Identifying individuals at high risk for diabetes: The atherosclerosis risk in communities study. Diabetes Care 28(8): 2013-2018.
- Dotevall A, Johansson S, Wilhelmsen L, Rosengren A (2004) Increased levels of triglycerides, BMI and blood pressure and low physical activity increase the risk of diabetes in Swedish women. A prospective 18-year follow-up of the BEDA study. Diabetic Medicine 21(6): 615-622.
- Mykkänen L, Haffner S, Rainwater D, Karhapää P, Miettinen H, et al. (1997) Relationship of LDL size to insulin sensitivity in normoglycemic men. Arteriosclerosis, Thrombosis, and Vascular Biology 17: 1447-1453.
- Zhao S, Yu S, Chi C, Fan X, Tang J, et al. (2019) Association between macro- and microvascular damage and the triglyceride glucose index in community-dwelling elderly individuals: The Northern Shanghai Study. Cardiovascular Diabetology 18(1): 95.
- Raghavan S, Vassy J, Ho Y, Song R, Gagnon D, et al. (2019) Diabetes mellitus–related all‐cause and cardiovascular mortality in a national cohort of adults. Journal of the American Heart Association 8(4): e011295.
- Balikai F, Deshpande N, Javali S, Shetty D, Benni J, et al. (2020) The relationship between serum triglyceride level and heart rate variability in type 2 diabetes mellitus patients of North Karnataka. Journal of Diabetology 11(3): 191-197.
- Ference B, Kastelein J, Ray K, Ginsberg H, Chapman M, et al. (2019) Association of triglyceride-lowering LPL variants and LDL-C–lowering LDLR variants with risk of coronary heart disease. JAMA 321(4): 364-373.
- Nordestgaard B (2016) Triglyceride-rich lipoproteins and atherosclerotic cardiovascular disease. Circulation Research 118(4): 547-563.
- Hjellvik V, Sakshaug S, Strom H (2012) Body mass index, triglycerides, glucose, and blood pressure as predictors of type 2 diabetes in a middle-aged Norwegian cohort of men and women. Clinical Epidemiology 4: 213-224.
- Balkau B, King H, Zimmet P, Raper L (1985) Factors associated with the development of diabetes in the micronesian population of Nauru. American Journal of Epidemiology 122(4): 594-605.
- McLaughlin T, Abbasi F, Cheal K, Chu J, Lamendola C, et al. (2003) Use of metabolic markers to identify overweight individuals who are insulin resistant. Annals of Internal Medicine 139(10): 802-809.
- Zheng D, Dou J, Liu G, Pan Y, Yan Y, et al. (2018) Association between triglyceride level and glycemic control among insulin-treated patients with type 2 diabetes. The Journal of Clinical Endocrinology & Metabolism 104(4): 1211-1220.
- Shaw J, Sicree R, Zimmet P (2010) Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Research and Clinical Practice 87(1): 4-14.
- Singh R, Gangadharappa H, Mruthunjaya K (2017) Phospholipids: Unique carriers for drug delivery systems. Journal of Drug Delivery Science and Technology 39: 166-179.
- Renne M, de Kroon A (2017) The role of phospholipid molecular species in determining the physical properties of yeast membranes. FEBS Letters 592(8): 1330-1345.
- Dickson R (1998) Sphingolipid functions in saccharomyces cerevisiae: Comparison to mammals. Annual Review of Biochemistry 67: 27-48.
- Farine L, Niemann M, Schneider A, Bütikofer P (2015) Phosphatidylethanolamine and phosphatidylcholine biosynthesis by the Kennedy pathway occurs at different sites in Trypanosoma brucei. Scientific Reports 5.
- Gibellini F, Smith T (2010) The Kennedy pathway-De novo synthesis of phosphatidylethanolamine and phosphatidylcholine. IUBMB Life 62(6): 414‐428.
- Kaur JA (2014) Comprehensive review on metabolic syndrome. Cardiology Research and Practice 2014: 943162.
- Roberts CK, Hevener AL, Barnard RJ (2013) Metabolic syndrome and insulin resistance: underlying causes and modification by exercise training. Compr Physiol 3(1): 1‐58.
- Martínez Ramírez M, Madero M, Vargas Alarcón G, Vargas Barrón J, Fragoso J, et al. (2016) HDL-sphingomyelin reduction after weight loss by an energy-restricted diet is associated with the improvement of lipid profile, blood pressure, and decrease of insulin resistance in overweight/obese patients. Clinica Chimica Acta 454: 77-81.
- Younsi M, Quilliot D, Al Makdissy N, Delbachian I, Drouin P, et al. Erythrocyte membrane phospholipid composition is related to hyperinsulinemia in obese nondiabetic women: Effects of weight loss. Metabolism 51(10): 1261-1268.
- Zeghari N, Vidal H, Younsi M, Ziegler O, Drouin P, et al. (2000) Adipocyte membrane phospholipids and PPAR-γ expression in obese women: relationship to hyperinsulinemia. American Journal of Physiology-Endocrinology and Metabolism 279(4): E736-E743.
- Zhao J, Zhu Y, Hyun N, Zeng D, Uppal K, et al. (2014) Novel metabolic markers for the risk of diabetes development in American Indians. Diabetes Care 38(2): 220-227.
- Borkman M, Storlien L, Pan D, Jenkins A, Chisholm D, et al. (1993) The relation between insulin sensitivity and the fatty-acid composition of skeletal-muscle phospholipids. New England Journal of Medicine 328(4): 238-244.
- Vessby B, Tengblad S, Lithell H (1994) Insulin sensitivity is related to the fatty acid composition of serum lipids and skeletal muscle phospholipids in 70-year-old men. Diabetologia 37(10): 1044-1050.
- Clore J, Harris P, Li J, Azzam A, Gill R, et al. (2000) Changes in phsophatidylcholine fatty acid composition are associated with altered skeletal muscle insulin responsiveness in normal man. Metabolism 49(2): 232-238.
- Semba R, Gonzalez Freire M, Moaddel R, Sun K, Fabbri E, et al. (2018) Altered plasma amino acids and lipids associated with abnormal glucose metabolism and insulin resistance in older adults. The Journal of Clinical Endocrinology & Metabolism 103(9): 3331-3339.
- Beeson M, Sajan M, Dizon M, Grebenev D, Gomez Daspet J, et al. (2003) Activation of protein kinase C- by insulin and phosphatidylinositol-3,4,5-(PO4)3 is defective in muscle in type 2 diabetes and impaired glucose tolerance: Amelioration by rosiglitazone and exercise. Diabetes 52(8): 1926-1934.
- Toledo F, Menshikova E, Ritov V, Azuma K, Radikova Z, et al. (2007) Effects of physical activity and weight loss on skeletal muscle mitochondria and relationship with glucose control in type 2 diabetes. Diabetes 56(8): 2142-2147.
- Coen P, Menshikova E, Distefano G, Zheng D, Tanner C, et al. (2015) Exercise and weight loss improve muscle mitochondrial respiration, lipid partitioning, and insulin sensitivity after gastric bypass surgery. Diabetes 64(11): 3737-3750.
- Demopoulos C, Pinckard R, Hanahan D (1979) Platelet-activating factor. Evidence for 1-O-alkyl-2-acetyl-sn-glyceryl-3-phosphorylcholine as the active component (a new class of lipid chemical mediators). Journal of Biological Chemistry 254(19): 9355-9358.
- Rosenson R, Stafforini D (2012) Modulation of oxidative stress, inflammation, and atherosclerosis by lipoprotein-associated phospholipase A2. Journal of Lipid Research 53(9): 1767-1782.
- Bochkov V, Gesslbauer B, Mauerhofer C, Philippova M, Erne P, et al. (2017) Pleiotropic effects of oxidized phospholipids. Free Radical Biology and Medicine 111: 6-24.
- Fu P, Birukov K (2009) Oxidized phospholipids in control of inflammation and endothelial barrier. Translational Research 153(4): 166-176.
- Spinas E, Kritas S, Saggini A, Mobili A, Caraffa A, et al. (2014) Role of mast cells in atherosclerosis: A classical inflammatory disease. International Journal of Immunopathology and Pharmacology 27(4): 517-521.
- Ersoy B, Huseyinov A, Darcan S (2005) The role of platelet-activating factor in pathogenesis of type 1 diabetes. Diabetes Care 28(4): 980-980.
- Trapali M, Mavri Vavayanni M, Siafaka Kapadai A (1996) PAF-acetylhydrolase activity and PAF levels in pancreas and plasma of well-fed, diabetic and fasted rat. Life Sciences 59(10): 849-857.
- Piuri G, Basello K, Rossi G, Soldavini C, Duiella S, et al. (2020) Methylglyoxal, glycated albumin, PAF, and TNF-α: Possible inflammatory and metabolic biomarkers for management of gestational diabetes. Nutrients 12(2): 479.
- Gomes M, Cobas R, Nunes E, Nery M, Neto CFH, et al. (2008) Serum platelet-activating factor acetylhydrolase activity: A novel potential inflammatory marker in type 1 diabetes. Prostaglandins & Other Lipid Mediators 87(1-4): 42-46.
- Ulijaszek S (2003) Obesity: Preventing and managing the global epidemic. Report of a WHO Consultation. WHO Technical Report Series.
- (2002) Third Report of the National Cholesterol Education Program (NCEP) Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation 106: 3143-3143.
- Tomlinson J, Tchernof A (2010) Special issue on steroids in obesity and diabetes. The Journal of Steroid Biochemistry and Molecular Biology 122(1-3): 1-2.
- Hwang J, Weiss R (2014) Steroid-induced diabetes: A clinical and molecular approach to understanding and treatment. Diabetes/Metabolism Research and Reviews 30(2): 96-102.
- Gathercole L, Stewart P (2010) Targeting the pre-receptor metabolism of cortisol as a novel therapy in obesity and diabetes. The Journal of Steroid Biochemistry and Molecular Biology 122(1-3): 21-27.
- Brown L, Clegg D (2010) Central effects of estradiol in the regulation of food intake, body weight, and adiposity. The Journal of Steroid Biochemistry and Molecular Biology 122(1-3): 65-73.
- Foryst Ludwig A, Kintscher U (2010) Metabolic impact of estrogen signalling through ERalpha and ERbeta. The Journal of Steroid Biochemistry and Molecular Biology 122(1-3): 74-81.
- Zoth N, Weigt C, Laudenbach Leschowski U, Diel P (2010) Physical activity and estrogen treatment reduce visceral body fat and serum levels of leptin in an additive manner in a diet induced animal model of obesity. The Journal of Steroid Biochemistry and Molecular Biology 122(1-3): 100-105.
- Habib G (2009) Systemic effects of intra-articular corticosteroids. Clinical Rheumatology 28(7): 749-756.
© 2021 Trapali M. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






