- Submissions

Full Text
Novel Research in Sciences
Clinical Characteristics and Outcomes of Patients with Diabetes and COVID-19
Xingang Li1,2, Yanli Xu1, Meihua Song1, Di Tian1, Rui Song1 and Zhihai Chen1*
1Center of Infectious Diseases, Beijing Ditan Hospital, Capital Medical University, Beijing 100015, China
2Department of Endocrinology, Beijing Ditan Hospital, Capital Medical University, Beijing 100015, China
*Corresponding author: Zhihai Chen, Center of Infectious Diseases, Beijing Ditan Hospital, Capital Medical University, Beijing, China
Submission: June 14, 2021;Published: June 28, 2021
.jpg)
Volume8 Issue2June, 2021
Abstract
Objective: The aim of this study is to investigate the risk factors relation to developing severe or critical cases in COVID-19 patients complicated with diabetes mellitus.
Methods: A single center, retrospective, and observational study was used to collect 26 inpatients diagnosed with COVID-19 and Diabetes. 17 and 9 patients were divided into the moderate and severe/ critical cases group. Demographic data and laboratory test results, clinical outcomes were collected, and the t-test was used for comparison between the groups.
Results: There was no statistically significant difference in age and body mass index between the two groups (54±8.2 vs. 61±9.1, p=0.19; 24.9±3.6 vs. 24.2±3.7, p=0.66). Fever (100% VS 47.1%) was the most common symptom; 5 cases of the moderate cases group were asymptomatic on admission. severe/critical cases group had more prominent laboratory abnormalities (NE%, LY, LY%, SAA, CRP, HBDH) as compared with moderate cases [(58.98±7.7 vs 71.69±11.53 p=0.02; 1.87±0.65 vs 0.99±0.27 p=0.01; 33.26±7.57 vs 21.61±10.2 p=0.03; 64.51±75.36 vs 148.61±121.49 p=0; 15. 24±15.42 vs 55.44±61.78 p=0.01; 156.56±48.49 vs 249.89±88.72 p=0.01)]. There were no statistically significant differences in the days of negative for SARS-CoV2 RNA and hospital stay between the two groups (28.94±10.28 vs. 25.33±7.17 p=0.35; 32.35±9.37 vs. 34.88±12.03 p=0.97)].
Conclusion: Laboratory abnormalities (NE%, LY, LY%, SAA, CRP, HBDH) may be helpful to identify critically ill patients of COVID-19 and diabetes early and reduce their mortality. Caution should be taken to patients with COVID-19 complicated with diabetes, especially with prominent laboratory results.
Keywords:COVID-19; Diabetes mellitus; Laboratory abnormalities
Introduction
COVID-19 (Coronavirus Infectious Disease 2019) caused by severe acute respiratory syndrome Coronavirus-2 (SARS-CoV-2) was upgraded to pandemic on March 11, 2020. As of February 25, 2021, more than 100 million have been documented worldwide, accounting for nearly 2500 thousand deaths. The total death rate of COVID-19 was 0.7% in Germany, while 10.8% in Italy [1]. Data followed soon after showed that COVID-19 patients with diabetes had a poor prognosis and higher mortality. Studies have also found patients with COVID-19 complicated with concomitant diseases, such as diabetes mellitus, were prone to develop severe cases and even had increased mortality [2]; nevertheless, older adults (>60 years) have a poor prognosis [3]. A recent study from the United States, involving 1122 patients with COVID-19, found that diabetes was related to a fourfold increase in mortality [4]. However, clinical features of diabetes with COVID-19 are still incomplete and evolving, especially about the risk factors of progression of patients with diabetes and COVID-19. In the current study, we retrospectively reviewed 26 inpatients diagnosed with COVID-19 complicated with diabetes admitted to Beijing Ditan Hospital. We explored the high-risk factors of COVID-19 patients with diabetes progressing to critical illness and improved clinical outcomes.
Methods
Data sources
A single-center, retrospective, and observational study were used to collect 26 inpatients diagnosed with COVID-19 complicated with diabetes admitted to Beijing Ditan Hospital, from January 20, 2020, to June 30, 2020. Demographic data, symptoms, laboratory tests, comorbidities, treatments, and clinical outcomes have been collected. The patients were divided into the moderate cases group and severe/ critical cases group.
Case definitions
The inclusion procedures and criteria were by the Guidelines for COVID-19 Diagnosis and Treatment (Trial version 7) [5] published by the National Health Commission of the People’s Republic of China.
Clinical classification
Moderate cases: Showing fever and respiratory symptoms with radiological findings of pneumonia.
Severe cases: Cases meeting any of the following criteria:
1) Respiratory distress (≧30 breaths/ min)
2) Finger oxygen saturation≤93% at rest
3) Arterial partial pressure of oxygen (PaO2)/ fraction of inspired oxygen (FiO2) ≦300mmHg (l mmHg=0.133kPa); PaO2/ FiO2 in high-altitude areas (at an altitude of over 1,000 meters above the sea level) shall be corrected by the following formula: PaO2/ FiO2 ×[Atmospheric pressure (mmHg)/760]
4) Cases with chest imaging that showed obvious lesion progression within 24-48 hours >50% shall be managed as severe cases.
Critical cases: cases are meeting any of the following criteria: 1. Respiratory failure and requiring mechanical ventilation; 2. Shock; 3. With other organ failure that requires ICU care.
Statistical analysis
Categorical variables were described as frequency rates and percentages, and continuous variables were described using the means and standard deviations. Means for continuous variables were compared using independent group t-tests when the data were normally distributed; all tests were 2-sided with P < 0.05 as the significance threshold. The analysis was performed with SPSS 26.0.
Results
Demographic and clinical characteristics
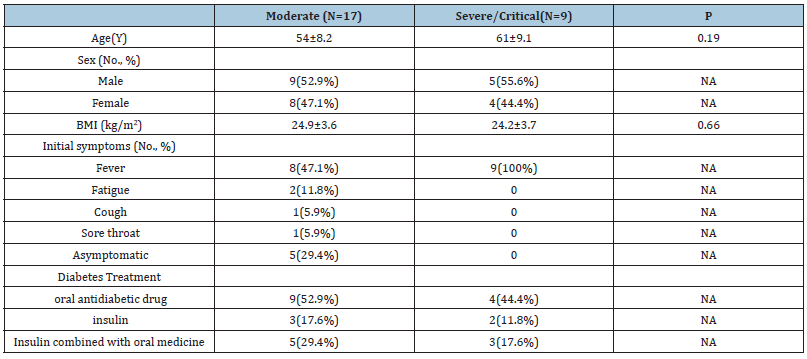
Of all 26 patients, 17 and 9 patients were categorized into the moderate and severe/ critical cases group, respectively. There was no statistically significant difference in age and body mass index between the two groups (54±8.2 vs. 61±9.1, p=0.19; 24.9±3.6 vs. 24.2±3.7, p=0.66). Fever (100% VS 47.1%) was the most common symptom. Moreover, 5 cases of the moderate cases group were asymptomatic on admission. 29.4% cases of severe/ critical cases group received with insulin or insulin combined with oral medicine. In contrast, 47% of cases of the moderate cases group were treated with insulin or insulin combined with an oral medicine (Table 1).
Table 1: Demographic and clinical characteristics of the patients.
Laboratory findings on admission
Results of laboratory tests on admission are shown in Table 2. severe/ critical cases group had more prominent laboratory abnormalities (NE%, LY, LY%, SAA, CRP, HBDH) as compared with moderate cases [(58.98±7.7 vs 71.69±11.53 p=0.02; 1.87±0.65 vs 0.99±0.27 p=0.01; 33.26±7.57 vs 21.61±10.2 p=0.03; 64.51±75.36 vs 148.61±121.49 p=0; 15.24±15.42 vs 55.44±61.78 p=0.01; 156.56±48.49 vs 249.89±88.72 p=0.01)]. There were, however, no marked differences in the remaining indexes between the two groups (all P>0.05). (Table 2).
Treatment, complications, and clinical outcomes
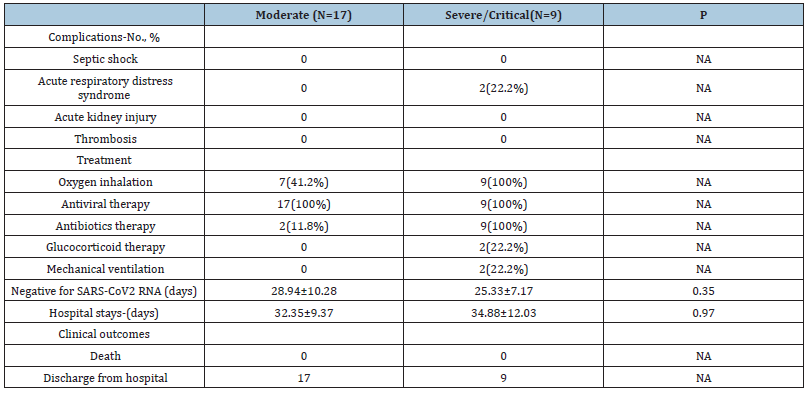
The organ dysfunction and treatment of the 26 patients are shown in Table 3. All patients received antiviral therapy (lopinavirritonavir and alpha-interferon). In the severe/ critical cases group, 9 cases received oxygen and antiviral therapy. 7(41.2%) cases received oxygen inhalation, and 2(11.8%) cases received antibiotics therapy in the moderate cases group. 2 critical cases yielded ARDS, then received systemic corticosteroid and mechanical ventilation. All patients had been discharged from hospital. There were no marked differences in the days of Negative for SARS-CoV2 RNA and Hospital stay between the two groups (28.94±10.28 vs. 25.33±7.17 p=0.35; 32.35±9.37 vs. 34.88±12.03 p=0.97)] (Table 3).
Table 2: Laboratory findings of patients on admission to hospital.
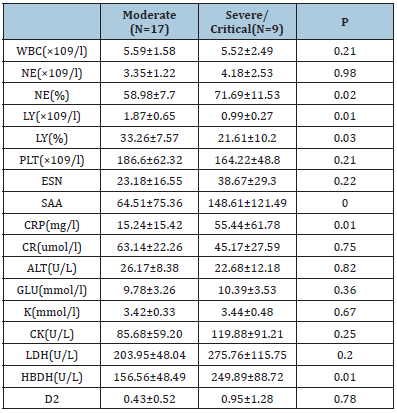
Abbreviations:
WBC: White Blood Cell; NE: Neutrophil; LY: Lymphocyle; PLT: Platelet; ESN: Erythrocyte Sedimentation Rate; CR: Creatinine; CRP: C-Reactive Protein; SAA: Serum Amyloid A; K: potassium; CK: Creatine Kinase; LDH: Lactate Dehydrogenase; GLU: Glucose; D2: D2 Polymer; HBDH: Hydroxybutyrate Dehydrogenase; ALT: Alanine Aminotransferase.
Table 3: Treatments and outcomes of Patients Infected with COVID-19.
Discussion
COVID-19 and diabetes are very popular and interact with each other. In an Early epidemiological study in Wuhan, Huang et al. [6] found that 8 patients had diabetes among 41 COVID-19 patients; the following retrospective studies confirmed the high percentage of diabetes that varied from 7 to 21% [7]. In good agreement with early case reports, some studies found a prevalence of COVID-19 patients complicated with diabetes varied from 8% to 10% [8- 11]. Hu et al. [11] also reported the percentage of critical cases in diabetes and hypertension was 44.5 % and 41.7 %, respectively. Furthermore, three meta-analyses [12-14] have reported COVID-19 patients with diabetes are at greater risk of receiving ICU treatment and have a higher risk of dying during hospitalization. Zhu et al. [15] found that all-cause mortality in patients with diabetes increased a significant 1.5-fold even after adjustment for other confounding factors, such as obesity, race/ ethnicity, age and sex, in contrast to the groups without diabetes. But when we interpret these results, we need to think more about them. Diabetes is a common chronic disease, and its incidence rate and morbidity rate are increasing very fast. Li et al. [16] reported that the morbidity rate of diabetes in Chinese adults is 12.8%. Previous studies, especially those from China, showed that the proportion of diabetic patients in COVID-19 is very close to Li and colleagues’ results. It is still uncertain that diabetes has a higher susceptibility to COVID-19. For now, though, some marked differences between diabetes patients with and those without developing critical illness should be observed.
Reports published previously have described the laboratory results of COVID-19. Guan et al. [17] reported that serious laboratory abnormalities including leukopenia, lymphopenia are more prominent in severe cases. Guo et al. [18] also reported that COVID-19 cases with diabetes(n=37) had more prominent laboratory abnormalities (i.e., lymphocyte, red blood cells, hemoglobin, neutrophils, erythrocyte sedimentation rate, D-dimer) as compared to the cohorts without diabetes (n=137). Zhu et al. [15] compared the laboratory profile of 7337 patients of COVID-19, of which 810 had diabetes compared to 6385 without diabetes. A marked decrease in lymphocyte count and a significant increase in leukocytosis, neutrophilia, D-dimer, ferritin, CRP, PCT, ALT, creatinine was observed in patients with diabetes, compared to the cohorts without diabetes (all p < 0.001). In our study, 26 inpatients were diagnosed with COVID-19 complicated with diabetes, and nine patients progressed to severe or critical cases during hospitalization.
There were, however, no marked differences in the Fasting blood glucose between the two groups. We found a significant difference in NE%, LY, LY%, SAA, CRP, and HBDH between the two groups on admission. A marked decrease in NE%, LY, LY% and a significant increase in SAA, CRP, HBDH were observed in the Severe/ Critical cases group, compared to the Moderate cases group (all p < 0.05). Laboratory abnormalities (NE%, LY, LY%, SAA, CRP, HBDH) can be used as indicators of disease progression during hospitalization, which would help identify critically ill patients of COVID-19 early and reduce their mortality. Li et al. [19], in a study of 25 death cases, found a marked decline of lymphocyte counts and the increase of NE, SAA, PCT, CRP, TNI, D-dimer, LDH, lactate can help to discover disease progression. Chen et al. [20] reported that CRP is an independent risk factor for adverse outcomes in patients with COVID-19 and diabetes mellitus, which may be helpful for early identification of critical patients with COVID-19. Fortunately, all patients in our study had recovered and discharged from the hospital. The sample size is a possible explanation for no difference in clinical outcomes in our study.
Recently, some studies have reported that diabetes can affect the progression of clinical course and prognosis of patients with COVID-19. The possible mechanisms were as follows: inflammation, Hypercoagulable state, activation of RAAS system and disorder of the Sympathetic Nervous System (SNS) [21]. Diabetes is a chronic hyperglycemia state, which is usually accompanied by abnormal metabolic indicators. Oxidative stress can affect the body’s response to the invasion of external antigens [22]. Whether people with diabetes have inflammatory storm is currently unknown, but there is a consensus that immune-mediated inflammation is involved in the pathophysiological process of COVID-19. Some regretful but important lessons have been learned from SARS and MERS. Cytokine-mediated inflammatory response plays an important role in the pathogenesis of SARS and MERS. The delayed but prolonged systematic inflammation can be observed in histological examination of diabetic mice [23]. Diabetes promotes increased synthesis of glycosylation end products (AGEs) and proinflammatory cytokines, oxidative stress, in addition to impairing T-cell mediated immune response and altering cytokine production [24].
The inflammatory status mentioned above may trigger the coagulation cascade. Certain cytokines could increase levels of clotting factors and relative inhibition of the fibrinolytic system. Cao et al. [25] reported that COVID-19 patients exhibited a hypercoagulable state, and some patients even progress to overt Disseminated Intravascular Coagulation (DIC). Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which binds to Angiotensin-Converting Enzyme 2 (ACE2), was expressed in different metabolic organs and tissues, including the pancreas [26]. The COVIDIAB project has launched the registration of COVID-19 with diabetes mellitus in the world (covidiab.e-dendrite.com), exploring the complex pathophysiology of COVID-19 with diabetes [27]. The abnormal response of cytokines and immune cells in patients with diabetes leads to the disorder of the immune system [28], and the possible mechanism is that hyperglycemia inhibits the ability of lymphocytes to respond to external stimuli [29]. Rimesh et al. [30] suggested that the interaction between COVID-19 and diabetes forms a vicious circle, which leads to the increase of blood glucose, in turn, the increased blood sugar affects the prognosis of covid-19.
Despite the fact that several studies have been conducted the high-risk factors and potential mechanisms of progression to severe or critical severity in patients with COVID-19 and diabetes mellitus, no clear conclusion has been drawn. Future studies need to explore the susceptibility of patients with diabetes, the effect of blood glucose on immune function and the mechanism of interaction between COVID-19 and diabetes, so as to provide guidance for clinical management.
Limitations
But due to time and condition limited, the findings are limited. First, the reader should bear in mind that the study is based on a small sample. Second, there are too few indicators including glycated hemoglobin, postprandial levels of plasma glucose, and C-peptide to describe diabetes, which can’t reflect the difference between the two groups and the impact on the endpoint. We next conduct a meta-analysis about the risk factors for the progression of Patients with Diabetes and COVID-19 to explore the effect of diabetes on COVID-19.
References
- Omer SB, Malani P, Rio C (2020) The COVID-19 pandemic in the US: a clinical update. JAMA 323(18): 1767-1768.
- Singh AK, Khunti K (2020) Assessment of risk, severity, mortality, glycemic control and antidiabetic agents in patients with diabetes and COVID-19: A narrative review. Diabetes Res Clin Pract 165: 108266.
- Pal R, Bhadada SK (2020) COVID-19 and non-communicable diseases. Postgrad Med 96(1137): 429-430.
- Bode B, Garrett V, Messler J, McFarland R, Crowe J, et al. (2020) Glycemic characteristics and clinical outcomes of COVID19 patients hospitalized in the United States. J Diabetes Sci Technol 14(4):813-821.
- NHC (2020) National Health Commission of the People's Republic of China. Notice on the issuance and dissemination of the Diagnosis and Treatment Protocol for COVID-19.
- Huang C, Wang Y, Li X, Ren L, Zhao J, et al. (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395(10223): 497-506.
- Singh AK, Khunti K (2020) Assessment of risk, severity, mortality, glycemic control and antidiabetic agents in patients with diabetes and COVID-19: A narrative review. Diabetes Res Clin Pract 165: 108266.
- Li B, Yang J, Zhao F, Zhi L, Wanf X, et al. (2020) Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol 109(5): 531-538.
- Yang J, Zheng Y, Gou X (2020) Prevalence of comorbidities in the novel Wuhan coronavirus (COVID-19) infection: a systematic review and meta-analysis. Int J Infect Dis.
- Emami A, Javanmardi F, Pirbonyeh N, Akbari A (2020) Prevalence of underlying diseases in hospitalized patients with COVID-19: a systematic review and meta-analysis. Arch Acad Emerg Med 8(1): e35.
- Hu Y, Sun J, Dai Z, Deng H, Xin L, et al. (2020) Prevalence and severity of coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. J Clin Virol 127: 104371.
- Roncon L, Zuin M, Rigatelli G, Zuliani G (2020) Diabetic patients with COVID-19 infection are at higher risk of ICU admission and poor short-term outcome. J Clin Virol 127: 104354.
- Kumar A, Arora A, Sharma O, Sharma P, Anikhindi SA, et al. (2020) Is diabetes mellitus associated with mortality and severity of COVID-19? A meta-analysis. Diabet Metabol Syndrome 14(4):535-545.
- Huang I, Lim MA, Pranata R (2020) Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia-A systematic review, meta-analysis, and meta-regression. Diabet Metabolic Syndrome 14(4): 395-403.
- Zhu L, She ZG, Cheng X, Guo J, Zhang BH, et al. (2020) Association of blood glucose control and outcomes in patients with COVID19 and pre-existing type 2 diabetes. Cell Metab 31(6): 1068-1077.
- Li Y, Teng D, Shi X, et al. (2020) Prevalence of diabetes recorded in mainland China using 2018 diagnostic criteria from the American diabetes association: national cross-sectional study. BMJ 369: m997.
- Guan WJ, Ni ZY, Hu Y, Liang WH, Chun O, et al. (2020) Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med 382(18): 1708-1720.
- Guo W, Li M, Dong Y, Zhou H, Zhang Z, et al. (2020) Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev p. e3319.
- Li X, Wang L, Yan S, Yang F, Xiang L, et al. (2020) Clinical characteristics of 25 death cases with COVID-19: A retrospective review of medical records in a single medical center, Wuhan, China. Int J Infect Dis 94: 128-132.
- Chen Y, Yang D, Cheng B, Chen J, Peng A, et al. (2020) Clinical characteristics and outcomes of patients with diabetes and covid-19 in association with glucose-lowering medication. Diabetes Care 43(7): 1399-1407.
- Tadic M, Cuspidi C, Sala C (2020) COVID-19 and diabetes: Is there enough evidence? J Clin Hypertens (Greenwich) 22(6): 943-948.
- Knapp Sylvia (2013) Diabetes and infection: is there a link? - A mini-review. Gerontology 59(2): 99-104.
- Kulcsar KA, Coleman CM, Beck SE, Frieman MB (2019) Comorbid diabetes results in immune dysregulation and enhanced disease severity following MERS-CoV infection. JCI Insight 4(20): e131774.
- John RP, Tomasz JG, Rhian MT (2018) Diabetes, hypertension, and cardiovascular disease: clinical insights and vascular mechanisms. Canadian J Cardiol 34(5): 575-584.
- Cao W, Li T (2020) COVID-19: towards understanding of pathogenesis. Cell Res 30(5): 367-369.
- Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, et al. (2004) Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus: a first step in understanding SARS pathogenesis. J Pathol 203(2): 631-637.
- Rubino F, Amiel SA, Zimmet P, Alberti G, Bornstein S, et al. (2020) New-onset diabetes in Covid-19. N Engl J Med 383(8): 789-790.
- Reading PC, Allison J, Crouch EC, Anders EM (1998) Increased susceptibility of diabetic mice to influenza virus infection: compromise of collectin-mediated host defense of the lung by glucose? Journal of Virology 72(8): 6884-6887.
- Moutschen MP, Scheen AJ, Lefebvre PJ (1992) Impaired immune responses in diabetes mellitus: analysis of the factors and mechanisms involved. Relevance to the increased susceptibility of diabetic patients to specific infections. Diabete Metab 18(3): 187-201.
- Pal R, Bhadada SK (2020) COVID-19 and diabetes mellitus: An unholy interaction of two pandemics. Diabetes Metab Syndr. 14(4): 513-517.
© 2021 Zhihai Chen. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






