- Submissions

Full Text
Novel Research in Sciences
Measurement of Vitamin D3 Metabolites in Liver and Serum After Treatment With The Consciousness Energy Healing Based Test Formulation in Vitamin D3 Deficiency Diet (VDD) Induced Animal Model
Mahendra Kumar Trivedi1*, Alice Branton1, Dahryn Trivedi1 and Snehasis Jana2
1Trivedi Global, Inc, Nevada, USA
2Trivedi Science Research Laboratory Pvt. Ltd., Thane (W), Maharashtra, India
*Corresponding author: Mahendra Kumar Trivedi, Trivedi Global, Inc, Nevada, USA
Submission: April 26, 2021;Published: May 3, 2021
.jpg)
Volume7 Issue4May, 2021
Abstract
The present study was evaluated the impact of Consciousness Energy Healing/Blessing Treatment (the Trivedi Effect®) on a novel test formulation in male Sprague Dawley (SD) rats. The animals were fed with Vitamin D3 Deficiency Diet (VDD) for the estimation of vitamin D3 metabolites such as 25-hydroxy cholecalciferol (25(OH) D3), and 1, 25-dihydroxy cholecalciferol (1, 25(OH)2 D3) in liver and serum samples; while cytochrome P450 enzymes (CYP24A and CYP27B) in liver homogenate. A novel proprietary test formulation was formulated including minerals (magnesium, zinc, copper, calcium, selenium, and iron), vitamins (ascorbic acid, pyridoxine HCl, alpha tocopherol, cyanocobalamin, and cholecalciferol), Panax ginseng extract, β-carotene, and cannabidiol isolate. The test formulation was divided into two parts; one part was defined as untreated test formulation, while the other parts of test formulation and three groups of the animals received Biofield Energy Healing Treatment by a renowned Biofield Energy Healer, Mr. Mahendra Kumar Trivedi. The expression of vitamin D3 metabolites like 25(OH) D3 level in liver homogenate was significantly (p≤0.001) increased by 184.83% in the Vitamin D Deficient (VDD) Diet + Biofield Energy Treatment per se to animals from day -15 (G6) group as compared to the VDD + untreated test formulation group (G4). Moreover, the level of 1, 25(OH)2 D3 was significantly increased by 18.28% and 32.83% in the G6 and VDD + Biofield Energy Treatment per se animals plus untreated test formulation (G9) groups, respectively as compared to the VDD + 0.5% CMC (G2) group. The expression of cytochrome P450 family 24 subfamily A member (CYP24A) in liver homogenate was significantly increased by 20.2% and 18.78% in the VDD + Biofield Energy Treated test formulation (G5) and VDD + Biofield Energy Treated test formulation from day -15 (G7) groups, respectively as compared to the G2 group. Additionally, the level of CYP27B expression was significantly increased by 12.45% and 13.78% in the G5 and VDD + Biofield Energy Treatment per se plus Biofield Energy Treated test formulation from day -15 (G8) groups, respectively as compared to the G2 group. Further, in serum the level of 25(OH) D3 was significantly (p≤0.001) increased by 7401.14%, 8976.1%, 10838.65%, and 9958.1% in the G5, G7, G8, and G9 groups, respectively as compared to the G2 group. Moreover, the level of 1, 25(OH)2 D3 was significantly (p≤0.001) increased by 186.26%, 167.99%, 130.54%, and 166.40% in the G5, G7, G8, and G9 groups, respectively as compared to the G2 group. Altogether, the Biofield Treated test formulation and Biofield Energy Treatment per se significantly increased the expression of vitamin D3 metabolites and mitochondrial enzyme functions. Thus, it could be helpful for the management of bone metabolic disorders, tumors, cardiovascular diseases, neuropsychiatric disorders, autoimmune diseases, and diabetes. Overall, the results showed the significant slowdown the disease progression and disease-related all other complications/symptoms in the preventive Biofield Energy Treatment group per se and/or Biofield Energy Treated Test formulation groups (viz. G6, G7, G8, and G9) compared with the disease control group.
Keywords: Biofield energy treatment; Vitamin D3 Metabolites; The trivedi effect®; Vitamin D3 deficiency diet; Calcitriol; CYP24A; CYP27B
Introduction
Vitamin D is one of the crucial nutrients to sustain the human health. Deficiency of 25 hydroxy vitamin D3 (25-OHD3) is highly prevalent in the world [1]. Chronic deficiency of vitamin D metabolite leads to inadequate mineralization of bone causes rickets in children and osteomalacia and osteoporosis in men and women, also increased risk of various chronic diseases, ranging from hypertension to diabetes to cancer [2]. Sufficient levels of vitamin D active metabolite like 1,25-dihydroxyvitamin cholecalciferol (1,25(OH)2D3) maintain human serum calcium balance by increasing calcium uptake in the small intestine and improved bone formation [3,4]. Besides, it also maintains cell proliferation, differentiation, immune regulation, apoptosis, neurogenesis, and genome stability [1]. Conversion into the active metabolite (1,25(OH)2D3) from the precursor (vitamin D) by cytochrome P450 enzymes in the liver (CYP27A1 and CYP2R1) and the kidney (CYP27B1). CYP27B1 is tightly regulated by the plasma levels of calcium, phosphate, parathyroid hormone (PTH) and 1,25(OH)2D3 itself [5]. It has also recently been found that Vitamin D Receptors (VDRs) exist in a variety of cells, thus it has a biological effect on more than mineral metabolism. Some research studies on rats reported that the deficiency in Vitamin D Receptor (VDR) may develop hypertension. It could be associated with the activation of the Renin-Angiotensin-aldosterone System (RAS), as vitamin D acts as a negative regulator of renin synthesis [6].
Various pre-clinical and clinical trials have been focused to develop a novel formulation that works to improve the overall health. However, there is no such novel herbal-based test formulation was designed that can improve the overall organ health using cell based standard assays. There is currently no universally accepted test formulation, which improve the organ health biomarkers. With this respect, the novel test formulation was designed on the basis of best scientific literature, which is the combination of different minerals (selenium, zinc, iron, calcium, copper, and magnesium), vitamins (cyanocobalamin, ascorbic acid, pyridoxine HCl, alpha tocopherol, and cholecalciferol), β-carotene, cannabidiol isolate, and Panax ginseng extract. This formulation was designed for overall health against many pathological bone health conditions. Minerals and vitamins present in the test formulation provide significant physiological support [7-9]. In addition, Panax ginseng is one of the best reported medicinal plants that improve mental, physical abilities, cognitive health, and is potent immunomodulator [10,11], while biological importance of cannabidiol has been widely reported in many pharmacological actions [12,13]. The test formulation and the animals per se were treated with the Biofield Energy by Biofield Energy Healer and were studied for vitamin D3 metabolites in liver and serum samples.
In recent years, Biofield Energy Treatment was reported in several scientific reports and clinical trials for the useful effects in case of cervical cancer patients [14], massage therapy [15], and many more. Complementary and Alternative Medicine (CAM) therapies have been reported that Biofield Therapies (or Healing Modalities) as one of the best preferred model of treatment with several benefits to enhance physical, mental, and emotional human wellness. National Centre of Complementary and Integrative Health (NCCIH) has been recognized and accepted Biofield Energy Healing as a CAM along with other therapies such as deep breathing, yoga, Tai Chi, Qi Gong, chiropractic/osteopathic manipulation, meditation, massage, special diets, homeopathy, progressive relaxation, guided imagery, acupressure, acupuncture, relaxation techniques, hypnotherapy, healing touch, movement therapy, pilates, Ayurvedic medicine, traditional Chinese herbs and medicines, naturopathy, essential oils, aromatherapy, Reiki, and cranial sacral therapy. Human Biofield Energy has subtle energy that has the capacity to work in an effective manner [16,17]. Biofield Energy Healing Treatment (the Trivedi Effect®) results has been published in numerous peer-reviewed science journals with significant outcomes in many scientific fields on various models in the materials science [18,19], agriculture science [20], microbiology [21,22], biotechnology [23,24], and improved bioavailability of various compounds [25,26], skin health [27,28], nutraceuticals [29], cancer research [30], bone health [31-33], overall human health and wellness. The present study was designed to study the mechanistic biomarkers such as the estimation of endothelin-1 and nitric oxide in kidney, lungs, and artery using ELISA assay in male Sprague-Dawley rats in presence of VDD diet and novel test formulation, which was treated with Biofield Energy Treatment/Blessing by a renowned Biofield Energy Healer.
Materials and Method
Chemicals and reagents
Copper chloride, cyanocobalamin (vitamin B12), calcium chloride, vitamin E (α-Tocopherol), cholecalciferol (vitamin D3), iron (II) sulfate, and Sodium Carboxymethyl Cellulose (Na-CMC) were procured from Sigma-Aldrich, USA. Pyridoxine hydrochloride (vitamin B6), zinc chloride, magnesium (II) gluconate, calcitriol, and β-carotene (Retinol, Provit A) were purchased from TCI, Japan. Ascorbic acid (vitamin C) and sodium selenate were obtained from Alfa Aesar, India. Panax ginseng extract and cannabidiol isolate were obtained from Panacea Phytoextracts, India and Standard Hemp Company, USA, respectively. For the estimation of mechanistic biomarkers, specific ELISA kits were used such as for detection of endothelin-1 and Nitric Oxide (NO), which were procured from CUSABIO, USA and My BioSource, USA, respectively.
Maintenance of animal
Randomly breed male Sprague Dawley (SD) rats with body weight ranges from 200 to 300gm were used in this study. The animals were purchased from M/s. Vivo Bio Tech, Hyderabad, India. Animals were randomly divided into nine groups based on their body weights consist of 6 animals of each group. They were kept individually in sterilized polypropylene cages with stainless steel top grill having provision for holding pellet feed and drinking water bottle fitted with stainless steel sipper tube. The animals were maintained as per standard protocol throughout the experiment.
Consciousness energy healing strategies
Each ingredient of the test formulation was divided into two parts. One part of the test compound was not received any treatment and were defined as the untreated or control sample. The second part of the test formulation was received Biofield Energy Treatment/Blessing by a renowned Biofield Energy Healer, Mr. Mahendra Kumar Trivedi under laboratory conditions for ~3 minutes. Besides, three group of animals also received Biofield Energy Treatment/Blessing by Mr. Trivedi under similar laboratory conditions for ~3 minutes. The Blessing/Treatment was given to the test items remotely without touching in the laboratory of Dabur Research Foundation, near New Delhi, India. After that, the Biofield Energy Treated samples was kept in the similar sealed condition and used as per the study plan. In the same manner, the control test formulation group was subjected to “sham” healer for ~3 minutes, under the same laboratory conditions. The “sham” healer has not any knowledge about the Biofield Energy Treatment. The Biofield Energy Treated/Blessed animals were also taken back to experimental room for further proceedings.
Experimental procedure
Seven days after acclimatization, animals were randomized and grouped based on the body weight. Dosing for groups G7 and G8 were initiated on day -15 and continued till end of the experiment. However, G1 to G6 and G9 groups were dosed from day 1 till the end of experiment. All the animals except G1 group received Vitamin D3 Deficient Diet (VDD) daily to the end of the experiment. Three weeks after the initiation of induction of VDD, all the groups were dose with the respective formulations.
Preparation of tissue homogenate
About 100mg of the liver tissue homogenate was rinsed with 1X PBS, homogenized in 1ml of 1X PBS and stored overnight at -20 °C. After two freeze-thaw cycles were performed to break the cell membranes, the homogenates were centrifuged for 5 minutes at 5000g, at 2 to 8 °C. The supernatant was removed and assayed immediately. Alternatively, aliquot and store samples at -20 °C or -80 °C. Centrifuge the sample again after thawing before the assay. Avoid repeated freeze-thaw cycles.
Estimation of vitamin D3 metabolites 25(OH) D3, 1, 25(OH)2D3 in liver homogenate
Liver homogenate was subjected for the estimation of level of 25(OH) D3 and 1, 25(OH)2D3. Both the metabolites were estimated using ELISA method as per manufacturer’s recommended standard procedure [34].
Estimation of mRNA expression of CYP24A and CYP27B in liver homogenate
Liver homogenate was subjected for the estimation of level of mRNA expression of CYP24A and CYP27B. Both the genes were estimated using ELISA method as per manufacturer’s recommended standard procedure [35].
Estimation of vitamin D3 metabolites 25(OH) D3, 1, 25(OH)2 D3 in serum
Serum was subjected for the estimation of level of 25(OH) D3 and 1, 25(OH)2 D3. Both the metabolites were estimated using ELISA method as per manufacturer’s recommended standard procedure [36].
Statistical analysis
The data were represented as Mean ± Standard Error of Mean (SEM) and subjected to statistical analysis using Sigma-Plot statistical software (Version 11.0). For multiple comparison One-way analysis of variance (ANOVA) followed by post-hoc analysis by Dunnett’s test and for between two groups comparison Student’s t-test was performed. The p≤0.05 was considered as statistically significant.
Result and Discussion
Estimation of vitamin D3 metabolites 25(OH) D3, and 1,25(OH)2 D3 in liver homogenate
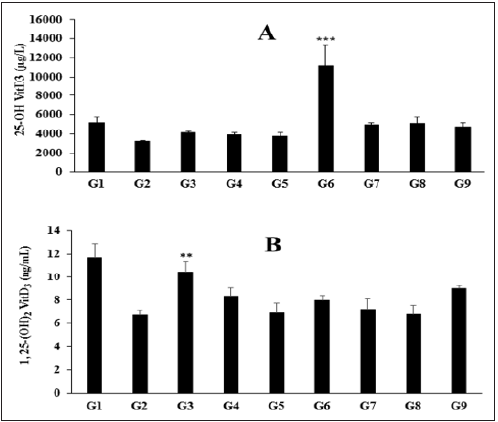
The effect of the Biofield Energy Treated test formulation on the level of vitamin D3 metabolites like 25-hydroxy vitamin D3 (25(OH) D3), and 1, 25 di-hydroxy vitamin D3 (1, 25(OH)2 D3) in liver homogenate is shown in Figure 1A and 1B, respectively. The normal control group (G1) showed the level of 25(OH) D3 and 1, 25(OH)2 D3 by (5151.66 ± 658.32µg/L) and (11.71 ± 1.15ng/ml), respectively. The positive control, calcitriol significantly increased the level of vitamin D metabolites 25(OH) D3 and 1, 25(OH)2 D3 by 27.10% and 55.72% (p≤0.01), respectively as compared to the G2 group. The level of 25(OH) D3 was significantly increased by 184.83% (p≤0.001), 24.53%, 29.51%, and 19.47% in the vitamin D deficient (VDD) diet + Biofield Energy Treatment per se to animals from day -15 (G6), VDD + Biofield Energy Treated test formulation from day -15 (G7), VDD + Biofield Energy Treatment per se plus Biofield Energy Treated test formulation from day -15 (G8), and VDD + Biofield Energy Treatment per se animals plus untreated test formulation (G9) groups, respectively as compared to the VDD + untreated test formulation group (G4). Besides, the level of 25(OH) D3 was significantly increased by 21.60%, 17.27%, 246.35%, 51.43%, 57.48%, 45.28% in the VDD + untreated test formulation (G4), VDD + Biofield Energy Treated test formulation (G5), G6, G7, G8, and G9, respectively as compared to the disease control (G2) group (VDD + 0.5% CMC) (Figure 1A). The level of 1, 25(OH)2 D3 was significantly increased by 23.48%, 18.28%, and 32.83% in the G4, G6, and G9 groups, respectively as compared to the G2 group (Figure 1B). Humans and animals obtain vitamin D3 (cholecalciferol) from dietary sources and from the skin is exposed to UV light. Vitamin D is metabolized by humans and animals to 25(OH)D and other metabolites. Therefore, animal tissue and animal-derived human foods contain 25-hydroxyvitamin D [25(OH)D] as well as the parent vitamin D. Evidence suggests that the content of 25(OH)D may be five times more potent than the content of parent vitamin D [37]. Thus, in this experiment, the Biofield Energy Treated test formulation has significantly increased the levels of both the metabolites, which might be more useful in the vitamin D deficient patients as well as healthy peoples to maintain good bone health.
Figure 1: Effect of the test formulation on the level of (A) 25 (OH) D3 and (B) 1, 25-(OH)2 D3 in liver homogenate of Sprague Dawley rats. G: Group; G1: Normal control (0.5% CMC); G2: Disease control (VDD: Vitamin D3 deficient diet + 0.5% CMC); G3: Reference item (VDD + Calcitriol); G4: (VDD + untreated test formulation); G5: (VDD + Biofield Energy Treated test formulation); G6: (VDD + Biofield Energy Treatment per se to animals from day -15; G7: (VDD + Biofield Energy Treated test formulation from day -15); G8: (VDD + Biofield Energy Treatment per se plus Biofield Energy Treated test formulation from day -15), and G9: (VDD + Biofield Energy Treatment per se animals plus untreated test formulation). Values are presented as mean ± SEM (n=6). **p≤0.01 vs. G2; ***p≤0.001 vs. G2 and G4 groups.
Estimation of mRNA expression of CYP24A and CYP27B in liver homogenate
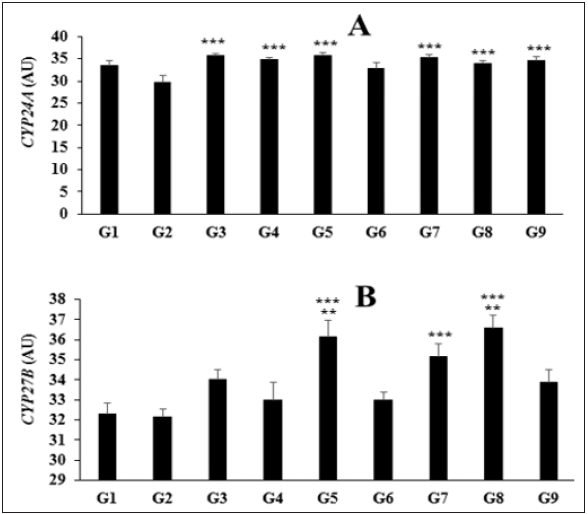
The effect of the test formulation on the expression of cytochrome P450 family 24 subfamily A member (CYP24A) and cytochrome P450 family 27 subfamily B member (CYP27B) in liver homogenate is shown in Figure 2A and 2B. The normal control group (G1) showed the expression of CYP24A and CYP27B by (33.43 ± 1.11µg/L) and (32.29 ± 0.57ng/mL), respectively. The positive control, calcitriol significantly increased the level of vitamin D metabolites 25(OH) D3, and 1, 25(OH)2 D3 by 27.10% and 55.72%, respectively as compared to the G2 group. The level of CYP24A expression was significantly (p≤0.001) increased by 17.33%, 20.2%, 10.1%, 18.78%, 13.97%, and 16.36% in the G4, G5, G6, G7, G8, and G9, respectively as compared to the disease control (G2) group (Figure 2A). Similarly, the level of CYP27B expression was significantly increased by 2.68%, 12.45%, 2.68%, 9.33%, and 13.78% in the G4, G5, G6, G7, and G8, respectively as compared to the disease control (G2) group. Moreover, expression of CYP27B was significantly increased by 9.52% (p≤0.01), 6.48%, and 10.82% (p≤0.01) in the G5, G7, and G8 groups, respectively as compared to the G4 group (Figure 2B). CYP24 enzyme plays the major role in fine-tuning the levels and actions of 1,25(OH)2 D3 at the tissue level. It is considered as one of the pivotal determinant of the biological half-life of 1,25(OH)2 D3. Apart from this, vitamin D analogs often stimulates the expression of CYP24 [38,39].
Figure 2: Effect of the test formulation on the level of (A) CYP24A and (B) CYP27B in liver homogenate of Sprague Dawley rats. G: Group; G1: Normal control (0.5% CMC); G2: Disease control (VDD: Vitamin D3 deficient diet + 0.5% CMC); G3: Reference item (VDD + Calcitriol); G4: (VDD + untreated test formulation); G5: (VDD + Biofield Energy Treated test formulation); G6: (VDD + Biofield Energy Treatment per se to animals from day -15; G7: (VDD + Biofield Energy Treated test formulation from day -15); G8: (VDD + Biofield Energy Treatment per se plus Biofield Energy Treated test formulation from day -15), and G9: (VDD + Biofield Energy Treatment per se animals plus untreated test formulation). Values are presented as mean ± SEM (n=6). ***p≤0.001 vs. G2 group; **p≤0.01 vs. G4 group.
Estimation of vitamin D3 metabolites 25(OH) D3, and 1, 25(OH)2 D3 in serum
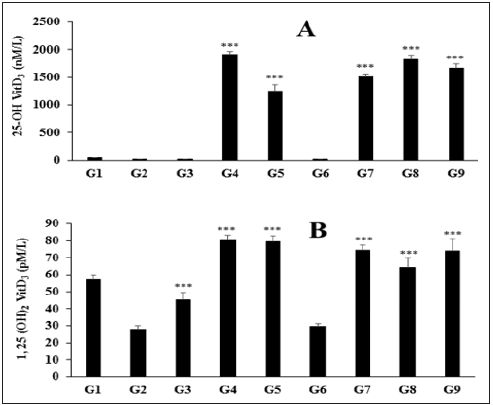
The effect of the Biofield Energy Treated test formulation on the level of vitamin D3 metabolites like 25-hydroxy vitamin D3 (25(OH) D3), and 1, 25 di-hydroxy vitamin D3 (1, 25(OH)2 D3) in serum is shown in Figure 3A and 3B. The normal control group (G1) showed the level of 25(OH) D3 and 1, 25(OH)2 D3 by (47.6 ± 4nM/L) and (57.1 ± 2.9pM/mL), respectively. The positive control, calcitriol significantly increased the level of vitamin D metabolites 25(OH) D3, and 1, 25(OH)2 D3 by 7.34% and 62.23%, respectively as compared to the G2 group. The level of 25(OH) D3 was significantly (p≤0.001) increased by 7401.14%, 8976.1%, 10838.65%, and 9958.1% in the vitamin D deficient (VDD) diet + Biofield Energy Treated test formulation (G5), VDD + Biofield Energy Treated test formulation from day -15 (G7), VDD + Biofield Energy Treatment per se plus Biofield Energy Treated test formulation from day -15 (G8), and VDD + Biofield Energy Treatment per se animals plus untreated test formulation (G9) as compared to the G2 (Figure 3A). The level of 1, 25(OH)2 D3 was significantly (p≤0.001) increased by 186.26%, 6.40%, 167.99%, 130.54%, and 166.40% in the vitamin D deficient (VDD) diet + Biofield Energy Treated test formulation (G5), Vitamin D Deficient (VDD) diet + Biofield Energy Treatment per se to animals from day -15 (G6), VDD + Biofield Energy Treated test formulation from day -15 (G7), VDD + Biofield Energy Treatment per se plus Biofield Energy Treated test formulation from day -15 (G8), and VDD + Biofield Energy Treatment per se animals plus untreated test formulation (G9) as compared to the G2 group (Figure 3B). It is necessary to metabolize vitamin D for its biological activity. Vitamin D is hydroxylated to 25(OH) D3 in the liver and then further hydroxylated in the kidney to produce 1,25(OH)2 D3. The metabolite, 1,25-(OH)2 D3 is considered as the active form of vitamin D responsible for bone-mineral mobilization and initiation of active intestinal absorption of calcium and phosphorus [40]. Thus, improved vitamin D3 metabolites can be beneficial against many disorders related to cancer, immune function, and cardiovascular disease in addition to the regulation of mineral balance and bone health [41].
Figure 3: Effect of the test formulation on the level of (A) 25-(OH) D3 and (B) 1, 25-(OH)2 D3 in serum of Sprague Dawley rats. G: Group; G1: Normal control (0.5% CMC); G2: Disease control (VDD: Vitamin D3 deficient diet + 0.5% CMC); G3: Reference item (VDD + Calcitriol); G4: (VDD + untreated test formulation); G5: (VDD + Biofield Energy Treated test formulation); G6: (VDD + Biofield Energy Treatment per se to animals from day -15; G7: (VDD + Biofield Energy Treated test formulation from day -15); G8: (VDD + Biofield Energy Treatment per se plus Biofield Energy Treated test formulation from day -15), and G9: (VDD + Biofield Energy Treatment per se animals plus untreated test formulation). Values are presented as mean ± SEM (n=6). ***p≤0.001 vs. G2 group.
Overall, results revealed a significant improved the levels of 25(OH) D3 and 1, 25-(OH)2 D3 in both liver and serum, and gene expression in liver homogenate. Thus, the present research plan defined four groups, which were considered as preventive maintenance groups viz. G6, G7, G8, and G9, where the Biofield Energy Treatment per se and/or Biofield Energy Treated Test formulation in combination was used as preventive maintenance group with respect to an improved vitamin D3 metabolites and liver mixed function oxygen enzymes. The results showed the significant slowdown of the disease progression, disease-related all other complications and also reduced the chances of disease susceptibility in these groups. Based on the overall data, it suggests that the Biofield Energy Healing Therapy was found to be most effective and benefited in order to prevent and protect from the occurrence of any type of bone-related diseases in rat model. It indicated that Biofield Energy Treatment can act as a preventive maintenance therapy to slowdown the disease progression and disease-related complications of the existing aliments that will ultimately improve the overall health and quality of life in human.
Conclusion
Based on the study outcomes, it was found that the expression of vitamin D3 metabolites like 25(OH) D3 was significantly increased by 184.83% in the Vitamin D Deficient (VDD) diet + Biofield Energy Treatment per se to animals from day -15 (G6) group as compared to the VDD + untreated test formulation group (G4) in liver homogenate. The expression of cytochrome P450 family 24 subfamily A member (CYP24A) was significantly increased by 20.2% in the VDD + Biofield Energy Treated test formulation (G5) group; while CYP27B level was increased by 13.78% in the VDD + Biofield Energy Treatment per se plus Biofield Energy Treated test formulation from day -15 (G8) group as compared to the disease control (VDD + 0.5% CMC) group (G2). Further, in serum the level of 25(OH) D3 was also significantly increased by 7401.14%, 8976.1%, 10838.65%, and 9958.1% in the G5, VDD + Biofield Energy Treated test formulation from day -15 (G7), G8, and VDD + Biofield Energy Treatment per se animals plus untreated test formulation (G9) groups, respectively as compared to the G2 group. Overall, results revealed that the Trivedi Effect® significantly increased the expression of vitamin D3 metabolites and mitochondrial enzyme functions by increasing cytochrome genes. Overall, it can be concluded Biofield Energy Healing Treatment (the Trivedi Effect®) per se showed best results with respect to different efficacy and biomarker parameters in the preventive treatment approach (-15 days) as compared to the other preventive maintenance groups (G7, G8, and G9) in rat model study. It also helped to slowdown the disease progression and disease-related complications of the overall animal’s health. The data suggested that Biofield Energy Treatment per se and/or Biofield Energy Treated Test formulation in combination would be the best treatment strategies in order to prevent and protect from the occurrence of any type of diseases. Therefore, the Biofield Energy Treatment might act as a preventive maintenance therapy in order to cure, or full restoration of health or improve the overall health and quality of life in human. This test formulation can also be used against other disorders such as systemic lupus erythematosus, fibromyalgia, Addison disease, multiple sclerosis, myasthenia gravis, pernicious anemia, aplastic anemia, psoriasis, rheumatoid arthritis, Crohn’s disease, vitiligo, chronic fatigue syndrome and alopecia areata, as well as inflammatory disorders such as ulcerative colitis, atherosclerosis, dermatitis, hepatitis, and diverticulitis. However, Biofield Energy Healing Treated test formulation and Biofield Energy Healing Treatment per se can also be used in the prevention of brain disorders such as Alzheimer’s disease, dementias, brain cancer, epilepsy and other seizure disorders, mental disorders, Parkinson’s and other movement disorders, stroke and Transient Ischemic Attack (TIA), and in the improvement of overall health and quality of life.
Acknowledgement
The authors are grateful to Dabur Research Foundation, Trivedi Science, Trivedi Global, Inc., and Trivedi Master Wellness for the assistance and support during the work.
References
- Wang H, Chen W, Li D, Yin X, Zhang X, et al. (2017) Vitamin D and chronic diseases. Aging Dis 8(3): 346-353.
- Heaney RP (2008) Vitamin D in Health and Disease. CJASN 3(5): 1535-1541.
- Cross HS, Pavelka M, Slavik J, Peterlik M (1992) Growth control of human colon cancer cells by vitamin D and calcium in vitro. J Natl Cancer Inst 84(17): 1355-1357.
- Tong WM, Bises G, Sheinin Y, Ellinger A, Genser D, et al. (1998) Establishment of primary cultures from human colonic tissue during tumor progression: Vitamin D responses and vitamin D receptor expression. Int J Cancer 75(3): 467-472.
- Tissandié E, Guéguen Y, Lobaccaro JM, Aigueperse J, Souidi M (2006) Vitamin D: Metabolism, regulation and associated diseases. Med Sci 22(12): 1095-100.
- Jeong HY, Park KM, Lee MJ, Yang DH, Kim SH, et al. (2017) Vitamin D and hypertension. Electrolyte Blood Press 15(1): 1-11.
- Byrne JH, Voogt M, Turner KM, Eyles DW, McGrath JJ, et al. (2013) The impact of adult vitamin D deficiency on behaviour and brain function in male Sprague-Dawley rats. PLoS One 8(8): e71593.
- Rayman MP (2000) The importance of selenium to human health. Lancet 356(9225): 233-241.
- Beard JL, Connor JR (2003) Iron status and neural functioning. Ann Rev Nutr 23: 41-58.
- Kang S, Min H (2012) Ginseng, the immunity boost: The effects of panax ginseng on immune system. J Ginseng Res 36(4): 354-368.
- Yang Y, Ren C, Zhang Y, Wu X (2017) Ginseng: A nonnegligible natural remedy for healthy aging. Aging Dis 8(6): 708-720.
- Peres FF, Lima AC, Hallak JEC, Crippa JA, Silva RH, et al. (2018) Cannabidiol as a promising strategy to treat and prevent movement disorders? Front Pharmacol 9: 482.
- Nagarkatti P, Pandey R, Rieder SA, Hegde VL, Nagarkatti M (2009) Cannabinoids as novel anti-inflammatory drugs. Future Med Chem 1(7): 1333-1349.
- Lutgendorf SK, Mullen E, Russell D, Degeest K, Jacobson G, et al. (2010) Preservation of immune function in cervical cancer patients during chemoradiation using a novel integrative approach. Brain Behav Immun 24(8): 1231-1240.
- Ironson G, Field T, Scafidi F (1996) Massage therapy is associated with enhancement of the immune system's cytotoxic capacity. Int J Neurosci 84(1-4): 205-217.
- Jain S, Hammerschlag R, Mills P, Cohen L, Krieger R, et al. (2015) Clinical studies of biofield therapies: Summary, methodological challenges, and recommendations. Glob Adv Health Med 4: 58-66.
- Rubik B (2002) The biofield hypothesis: Its biophysical basis and role in medicine. J Altern Complement Med 8(6): 703-717.
- Trivedi MK, Tallapragada RM (2008) A transcendental to changing metal powder characteristics. Met Powder Rep 63(9): 22-31.
- Trivedi MK, Nayak G, Patil S, Tallapragada RM, Latiyal O (2015) Studies of the atomic and crystalline characteristics of ceramic oxide nano powders after bio field treatment. Ind Eng Manage 4: 161.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Mondal SC, et al. (2015) Morphological characterization, quality, yield and DNA fingerprinting of biofield energy treated alphonso mango (Mangifera indica L.). Journal of Food and Nutrition Sciences 3(6): 245-250.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Charan S, et al. (2015) Phenotyping and 16S rDNA analysis after biofield treatment on Citrobacter braakii: A urinary pathogen. J Clin Med Genom 3: 129.
- Trivedi MK, Patil S, Shettigar H, Mondal SC, Jana S (2015) Evaluation of biofield modality on viral load of Hepatitis B and C viruses. J Antivir Antiretrovir 7(3): 083-088.
- Trivedi MK, Patil S, Shettigar H, Bairwa K, Jana S (2015) Phenotypic and biotypic characterization of Klebsiella oxytoca: An impact of biofield treatment. J Microb Biochem Technol 7: 203-206.
- Nayak G, Altekar N (2015) Effect of biofield treatment on plant growth and adaptation. J Environ Health Sci 1: 1-9.
- Branton A, Jana S (2017) The influence of energy of consciousness healing treatment on low bioavailable resveratrol in male Sprague Dawley rats. International Journal of Clinical and Developmental Anatomy 3(3): 9-15.
- Branton A, Jana S (2017) The use of novel and unique biofield energy healing treatment for the improvement of poorly bioavailable compound, berberine in male Sprague Dawley rats. American Journal of Clinical and Experimental Medicine 5(4): 138-144.
- Kinney JP, Trivedi MK, Branton A, Trivedi D, Nayak G, et al. (2017) Overall skin health potential of the biofield energy healing based herbomineral formulation using various skin parameters. American Journal of Life Sciences 5(2): 65-74.
- Singh J, Trivedi MK, Branton A, Trivedi D, Nayak G, et al. (2017) Consciousness energy healing treatment based herbomineral formulation: A safe and effective approach for skin health. American Journal of Pharmacology and Phytotherapy 2(1): 1-10.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Plikerd WD, et al. (2017) A Systematic study of the biofield energy healing treatment on physicochemical, thermal, structural, and behavioral properties of magnesium gluconate. International Journal of Bioorganic Chemistry 2(3): 135-145.
- Trivedi MK, Patil S, Shettigar H, Mondal SC, Jana S (2015) The potential impact of biofield treatment on human brain tumor cells: A time-lapse video microscopy. J Integr Oncol 4(3): 141.
- Anagnos D, Trivedi K, Branton A, Trivedi D, Nayak G, et al. (2018) Influence of biofield treated vitamin D3 on proliferation, differentiation, and maturation of bone-related parameters in MG-63 cell-line. International Journal of Biomedical Engineering and Clinical Science 4(1): 6-14.
- Lee AC, Trivedi K, Branton A, Trivedi D, Nayak G, et al. (2018) The potential benefits of biofield energy treated vitamin D3 on bone mineralization in human bone osteosarcoma cells (MG-63). International Journal of Nutrition and Food Sciences 7(1): 30-38.
- Stutheit ME, Trivedi K, Branton A, Trivedi D, Nayak G, et al. (2018) Biofield energy treated vitamin D3: Therapeutic implication on bone health using osteoblasts cells. American Journal of Life Sciences 6(1): 13-21.
- Hollis BW (1990) 25-Hydroxyvitamin D3-1 alpha-hydroxylase in porcine hepatic tissue: Subcellular localization to both mitochondria and microsomes. Proc Natl Acad Sci USA 87(16): 6009-6013.
- Joly JG, Doyon C, Peasant Y (1975) Cytochrome P-450 measurement in rat liver homogenate and microsomes. Its use for correction of microsomal losses incurred by differential centrifugation. Drug Metab Dispos 3(6): 577-586.
- Holick MF (2009) Vitamin D status: Measurement, interpretation, and clinical application. Ann Epidemiol 19(2): 73-78.
- Roseland JM, Patterson KY, Andrews KW, Phillips MM, Pehrsson PR, et al. (2016) Interlaboratory trial for measurement of vitamin D and 25-hydroxyvitamin D [25(OH)D] in foods and a dietary supplement using liquid chromatography-mass spectrometry. Journal of Agricultural and Food Chemistry 64(16): 3167-3175.
- Tashiro K, Abe T, Oue N, Yasui W, Ryoji M (2004) Characterization of vitamin D-mediated induction of the CYP 24 transcription. Mol Cell Endocrinol 226(1-2): 27-32.
- Chen KS, DeLuca HF (1995) Cloning of the human 1 alpha,25-dihydroxyvitamin D-3 24-hydroxylase gene promoter and identification of two vitamin D-responsive elements. Biochim Biophys Acta 1263(1): 1-9.
- Shepard RM, Horst RL, Hamstra AJ, Delucat HF (1979) Determination of vitamin D and its metabolites in plasma from normal and anephric man. Biochem J 182(1): 55-69.
- Cantorna MT, Zhao J, Yang L (2012) Vitamin D, invariant natural killer T-cells and experimental autoimmune disease. Proceedings of the Nutrition Society 71(1): 62-66.
© 2021 Mahendra Kumar Trivedi. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






