- Submissions

Full Text
Novel Research in Sciences
The Carbohydrate Periodization During Muscle Disuse
HS Fernandes*
Estácio de Sá College, Nutrition departament, Fortaleza, Ceará.
*Corresponding author:HS Fernandes, Estácio de Sá College, Nutrition departament, Fortaleza, Ceará.
Submission: March 11, 2021;Published: April 19, 2021
.jpg)
Volume7 Issue3April, 2021
Abstract
The recovery from injury or illness requires otherwise healthy individuals to undergo a period muscle disuse that despite its clinical relevance, their physiological mechanisms during muscle disuse are important and need be elucidated. Besides that, a regular consumption of meals, around 4 to 6 times a day, approximately between 3 and 4 hours is interesting the periodization carbohydrate consumption that can offer adaptations that prevent damage to lipid metabolism after meals, maintain insulin sensitivity, control the accumulation of visceral adiposity and try mantain the muscle glycogen store acquired during activities.
Keywords: Disuse; Muscle disuse; Carbohydrates; Carbohydrate periodization
Abbreviation: MPS: Muscle Protein Synthesis; mTOR: Mammalian Target of Rapamycin; ATP: Adenosine Triphosphate; AMPK: AMP-Activated Protein Kinase; FAK: Focal Adhesion Kinase; AKT: Protein Kinase B; Glucose Transporter; FOXO3: Forkhead Box Proteins; MYH7: slow isoform of MyHC
Introduction
The recovery from injury or illness requires otherwise healthy individuals to undergo a period muscle disuse and/or physical inactivity, during which can be rapid skeletal muscle atrophy and declines in functional and metabolic capacity occur [1]. Despite its clinical relevance, the physiological mechanisms during muscle disuse when consuming carbohydrates have not been fully elucidated. Currently, it is known that atrophy occurring after any injury is different from the muscle atrophy from disuse [2]. The present work deals with the physiological implications of disuse and it’s relationship with carbohydrate periodization to propose nutritional prescriptions that help athletes during this type of situation.
The Effects of Muscle Disuse on Muscle Adaptations
A study evaluated the effect of 14 days of rest on skeletal muscle satellite cell content and fiber-type atrophy in middle-aged adults founding percentage of type 2 fibers and satellite cell content were reduced due rapid inadequate adaptation of skeletal muscle to applied inactivity [3]. Another study with healthy young men, found in one week of disuse there were sharp losses in muscle volume and a decline of approximately 36% daily rates of MPS (Muscle Protein Synthesis) [4], which can impact suppression both in post-absorptive and post-prandial MPS [5]. One more study with healthy young men found that by reducing daily steps to 1500 steps combined with overeating, these participants increased visceral adiposity by 49% and decreased insulin sensitivity by 44% [6]. A other study also healthy young men, programmed approximately 1300 steps per day for 21 days and found a attenuation of postprandial lipid metabolism and insulin sensitivity, in addition to increases in intra-abdominal fat mass [7]. During disuse, the body loses adaptations built in training situations, attenuating myofibrillar protein synthesis, muscle volume and lipid metabolism after meals and when in overeating there is damage in sensitivity to insulin and increase visceral adiposity.
The Metabolic Influence of Carbohydrates and Muscle Glycogen on Adaptive Responses to Disuse
Lack of physical performance can affect the glycogen particles located inside the myofibrils, impairing volume of mitochondria plasticity in cells [8] and low availability of Carbohydrates (CHO) for more than three consecutive weeks can reduces oxidation rate CHO during exercise [9]. Several mechanisms seem to explain decrease in muscle fiber size during atrophy due to disuse, including increased protein degradation and suppression of muscle protein synthesis [10]. Application of force generated by muscle contraction, triggers a mechanical overload that is defined in the synthesis of proteins by signaling mTOR (Mammalian Target of Rapamycin) and phosphorylation of the ribosomal protein S6K (p70S6K) [11]. Thus, anabolic responses after exercise are regulated mainly by the mTOR pathway [12] and responses related to generation of ATP (Adenosine Triphosphate) will come mainly from the regulation generated through the AMPK (AMP-Activated Protein Kinase) pathway, in which it’s triggered by the decrease in muscle glycogen content [9]. Therefore, AMPK activity is signaled in energy deficit while inhibiting mTOR, in which it requires energy available for it’s activity [13]. In addition AMPK pathway conserves ATP content by inhibiting glycogen and protein biosynthesis pathways, reciprocally activating catabolic signaling (fat oxidation and glucose transport/ uptake) to restore cellular energy status [14]. Therefore, AMPK and mTOR integrate metabolic signals to regulate the function of integrins [15]. The specific isoforms of integrins and their specific functions are factors that can, depending on total amount or phosphorylated amount, result in capillary refraction that occurs with muscle atrophy due to disuse [16] and integrin signaling could impact apoptotic cascades [17] through inhibition of FAK (Focal Adhesion Kinase) and subsequent loss of mitochondrial membrane potential, in addition to increased production of free radicals [18]. α5 integrin is involved in mitochondrial depolarization, indicating control dependent on mitochondrial metabolism [15].
Thus, the molecular mechanisms of signal transduction triggered by the involvement of integrins in relation to mitochondrial metabolic response are linked to gene expression [19] for involve AKT (Protein Kinase B) signaling and to contribute to anabolic metabolism by interacting with the Glucose Transporter 1 (GLUT1) by inhibiting the protein that promotes GLUT1 endocytosis, resulting in increased glucose absorption [20], in which it’s a substrate for glycolytic muscle fibers that co-express the α7 and ILK integrins (Integrin-Linked Kinase) [21], providing support for understanding the α7 integrin-ILK-AKT pro-survival pathway with α7 integrin undergoing a 28% decrease in its mRNA after 5 days of disuse [22], [23], being link of conditions of disuse and their metabolic implications in skeletal muscle [24]. During disuse, the changes in AMPK activity due to paradoxical hyperphosphorylation of p70S6K it will induce proteolytic processes in muscle fiber [25], such as dephosphorylation of FOXO3 (Forkhead Box Proteins) and increased expression of main ubiquitin enzymes via proteasome [26], decreasing level of phosphorylation and AMPK activity and affecting nucleocytoplasmic traffic of class IIA (nuclear-cytoplasmic traffic of class IIa histone deacetylases), resulting in decreased expression of genes, such as MYH7 (slow isoform of MyHC), leading to less signaling of muscle protein synthesis [25]. In other words, some negative adaptations to disuse involve AMPK signaling turn is balanced by the amount of muscle glycogen [9], directly influenced by carbohydrates consumption in the diets as shown in Figure 1.
Figure 1: Skeletal muscle metabolism involving disuse and carbohydrates consumption.
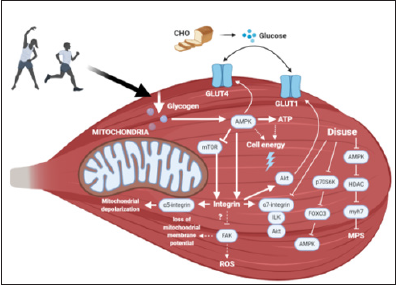
Carbohydrates Consumption in Disuse Situations
During disuse is most interesting thing would be a regular consumption of meals, around 4 to 6 times a day, approximately between 3 and 4 hours [27], [28] and one week of muscle disuse induces decline in daily myofibrillar protein synthesis rates mainly on skeletal muscle mRNA expression of genes involved in carbohydrate metabolismo [4]. That’s why, carbohydrates periodization proposal can be an alternative that benefits the athlete. So, Table 1 shows a model of carbohydrate comsuption periodization during one-week disuse with two days on high (7g/ kg of body weight (BW)/day), three days on moderate (5g/kg BW/ day) and two days on low carbohydrates consumption (3g/kg BW/ day).
Table 1: Example of CHO periodization during one week of disuse in g per kg of BW per.

Conclusion
Adequate and periodized carbohydrates consumption can offer adaptations that prevent damage to lipid metabolism after meals, maintain insulin sensitivity and control the accumulation of visceral adiposity. In addition to trying mantain the muscle glycogen store acquired during activities.
References
- ML Dirks, BT Wall, LJC Van Loon (2018) Interventional strategies to combat muscle disuse atrophy in humans: Focus on neuromuscular electrical stimulation and dietary protein. J Appl Physiol 125(3): 850-861.
- LK Lepley, SM Davi, JP Burland, AS Lepley (2020) Muscle atrophy after ACL injury: Implications for clinical practice. Sports Health 12(6): 579-586.
- Arentson LEJ, English KL, Paddon JD, Fry CS (2016) Fourteen days of bed rest induces a decline in satellite cell content and robust atrophy of skeletal muscle fibers in middle-aged adults. J Appl Physiol 120(8): 965-975.
- Kilroe SP, Jonathan F, Andrew MH, Sarah RJ, Benjamin PL, et al. (2019) Short-term muscle disuse induces a rapid and sustained decline in daily myofibrillar protein synthesis rates. Am J Physiol Endocrinol Metab 318(2): 117-130.
- Rudrappa SS, Wilkinson DJ, Greenhaff PL, Smith K, Idris I, et al. (2016) Human skeletal muscle disuse atrophy: Effects on muscle protein synthesis, breakdown, and insulin resistance - A qualitative review. Front Physiol 7(361): 1-10.
- Knudsen SH, Louise SH, Maria P, Thomas D, Jakob H, et al. (2012) Changes in insulin sensitivity precede changes in body composition during 14 days of step reduction combined with overfeeding in healthy young men. J Appl Physiol 113(1): 7-15.
- Olsen RH, Krogh MR, Carsten T, Frank WB, Bente KP (2014) Metabolic responses to reduced daily steps in healthy non exercising men. JAMA 299(11): 1261-1263.
- Nielsen J, Suetta C, Hvid LG, Schrøder HD, Aagaard P, et al. (2010) Subcellular localization-dependent decrements in skeletal muscle glycogen and mitochondria content following short-term disuse in young and old men. Am J Physiol Endocrinol Metab 299(6): 1053-1061.
- GL Close, Hamilton DL, Philp A, Burke LM, Morton JP (2016) New strategies in sport nutrition to increase exercise performance. Free Radic Biol Med 98: 144-158.
- Atherton PJ, Greenhaff PL, Phillips SM, Bodine SC, Adams CM, et al. (2016) Control of skeletal muscle atrophy in response to disuse: clinical/preclinical contentions and fallacies of evidence. Am J Physiol Endocrinol Metab 311(3): 594-604.
- Plant PJ, Dina B, Marie F, Tanya B, James B, et al. (2010) Cellular markers of muscle atrophy in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol 42(4): 461-471.
- Lee CH, Inoki K, Guan KL (2007) mTOR Pathway as a target in tissue hypertrophy. Annu Rev Pharmacol Toxicol 47(1): 443-467.
- Smiles WJ, Hawley JA, Donny MC, (2016) Effects of skeletal muscle energy availability on protein turnover responses to exercise. J Exp Biol 219(2): 214-225.
- Kahn BB, Alquier T, Carling D, Hardie DG (2005) AMP-activated protein kinase: Ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab 1(1): 15-25.
- R Ata, CN Antonescu (2017) Integrins and cell metabolism: An intimate relationship impacting cancer. Int J Mol Sci 18(1): 189.
- K Tyml, Mathieu OC (2001) Structural and functional changes in the microvasculature of disused skeletal. Front Biosci 6: 45-52.
- Stupack DG, Cheresh DA (2002) Get a ligand, get a life: Integrins, signaling and cell survival. J Cell Sci 115(19): 3729-3738.
- Visavadiya NP, Matthew PK, Vladislav R, Kalpita B, Cuihong J et al. (2016) Integrin-FAK signaling rapidly and potently promotes mitochondrial function through STAT3. Cell Commun Signal 14(1): 1-15.
- Werner E, Werb Z (2000) Integrins engage mitochondrial function for signal transduction by a mechanism dependent on Rho GTPases. J Cell Biol 158(2): 357-368.
- Hoxhaj G, Manning BD (2020) The PI3K-AKT network at the interface of oncogenic signaling and cancer metabolism. Nat Rev Cancer 20(2): 74-88.
- Mathes S, Vanmunster M, Bloch W, Suhr F (2019) Evidence for skeletal muscle fiber type ‑ specific expressions of mechanosensors. Cell Mol Life Sci 76(15):2987-3004.
- Boppart MD, Mahmassani ZS, Training A, City SL, Boppart MD, et al. (2019) Integrin signaling: linking mechanical stimulation to skeletal muscle hypertrophy. Am J Physiol Cell Physiol 317(4):629-641.
- Ziad MJD, S Mahmassani, Paul TR, Alec IM, Chris S, et al. (2019) Age-dependent skeletal muscle transcriptome response to bed rest-induced atrophy 2. J Appl Physiol 126(4): 894-902.
- Zou K, Benjamin MM, Brian J, Heather DH, Ziad M, et al. (2011) The α7β1 integrin increases muscle hypertrophy following multiple bouts of eccentric exercise. J Appl Physiol 111(4): 1134-1141.
- Vilchinskaya NA, Mochalova EP, Belova SP, Shenkman BS (2016) Dephosphorylation of amp-activated protein kinase in a postural muscle: A key signaling event on the first day of functional unloading. Complex Systems Biophysics 61(6): 1019-1025.
- Zhang S, Zhang Y, Li B, Chen N (2018) Physical inactivity induces the atrophy of skeletal muscle of rats through activating AMPK / FoxO3 signal pathway. Eur Rev Med Pharmacol Sci 22(1):199-209.
- Wall BT, Morton JP, Van Loon LJC (2015) Strategies to maintain skeletal muscle mass in the injured athlete: Nutritional considerations and exercise mimetics. Eur J Sport Sci 15(1): 53-62.
- Quintero KJ, Resende AS, Leite GSF, Lancha Junior AH (2018) An overview of nutritional strategies for recovery process in sports-related muscle injuries. Nutrire 43(1): 1-10.
© 2021 HS Fernandes. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






