- Submissions

Full Text
Novel Research in Sciences
Early Phenophases in Some Common Tree Species and Provenances in Western Norway During the Period 1964-2014 as Related to Mean January-April Temperatures, and Consequences of the Related Climate Change
Oddvar Skre*
Skre Nature and Environment, Norway
*Corresponding author:Skre O, Skre Nature and Environment, Norway
Submission: March 29, 2021;Published: April 15, 2021
.jpg)
Volume7 Issue2April, 2021
Abstract
The IPG garden at Fana (02) near Bergen, Norway was established in 1958, and during the years 1958-75 woody trees and shrubs of 26 species and provenances from all over Europe were transplanted to the site, 2 or 3 replicates of each. For comparison meteorological records of temperature and precipitation were collected from a nearby meteorological station. The mean January-April temperature at Fana increased from about 1.5 to 3.0 C during the observation period 1964-2014. Consequently, a significant trend against earlier budbreak was found during the period in the investigated species, and this change was closely correlated with increased temperature. The shift against earlier budbreak during the sample period was found to be 15-35 days depending on species and provenance, and the shift was stronger in species with late budbreak than in other species with early budbreak.
Introduction and Methods
The IPG garden at Fana (02) near Bergen, Norway was established in 1958 as one of 50 phenological gardens throughout Europe Figure 1, and during the years 1958-75 woody trees and shrubs of 26 European species and provenances [1] were transplanted to the site, 2 or 3 replicates of each. See Table 1 for origin and provenances. For comparison meteorological records of temperature and precipitation were collected from a nearby meteorological station. Due to ageing and storm felling a number of trees died during the observation period, and by 2013 only 17 species and provenances were left. The three spruce provenances were replaced by young trees from the same origin in 2000, because the old trees had grown too big, and were shadowing out other trees in the garden.
Figure 1: Map of Europe with locations of IPG gardens [1].
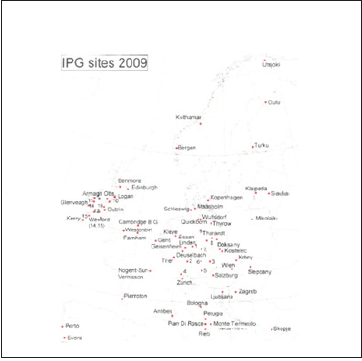
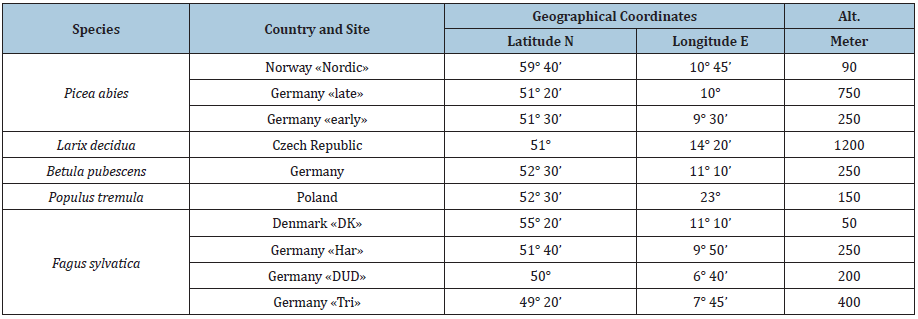
Table 1: Origin of species and provenances.
Tree species and provenances from the IPG gardens at Fana
and Kvithamar. Figure 1 were investigated for early phenophases as
compared with temperature records [2]. In another study [3] based
on earlier studies on a number of wild trees and herbs from various
parts of Fennoscandia were investigated for early phenophases as
compared with climatic parameters [4].
Generally, leaf fall and winter dormancy are induced mainly by
short days and is only slightly influenced by temperature, but when
the chilling requirements for dormancy breaking are fulfilled in
January or February, the buds are dehardened, and budbreak will
occur as soon as temperatures are high enough [5,6]. Because of the
raised winter temperatures, the risk of early dehardening and spring
frost damages in e.g. Betula pubescens is increased, this was also
confirmed in the present phenological study and in related studies
on birch [7,8]. The spring phenophases are therefore much more
influenced by temperature than the time for leaf fall and dormancy
induction [9]. Other studies, e.g., on birch (Betula pubescens) have
shown that the chilling requirements for dormancy breaking are
lower in northern and inland populations than in southern and
coastal populations of the same species, leading to earlier budbreak
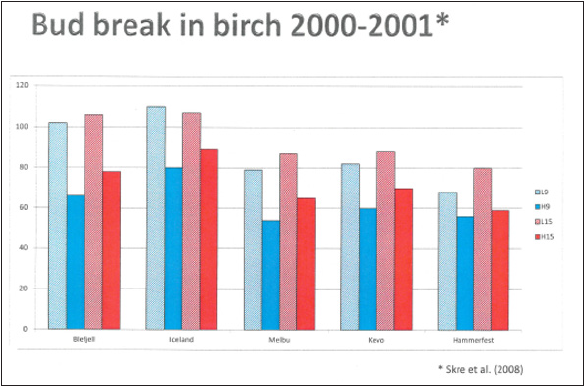
[5,10]. It is also found that high temperaturres during hardening in
fall would lead to delayed budbreak occurrence in spring (Figure
2).
Figure 2: Mean date of budbreak 2000-2001 (Julian days) in five birch populations from tree line localities in Fennoscandia ranging from 60 °N(Blefjell) to 71 °N(Hammerfest). The seedlings have been grown in greenhouse at high (L15) and low (L9) temperatures during the fall, followed by high (H15) and low (H9) winter temperatures [9].
For comparison with early phenophases, the mean January- April temperature was chosen rather than the mean temperature of March-May used by Nordli et al. [2] because the study included also some inland stations like Kvithamar with 3-4 °C lower winter temperatures, and consequently 1-2 months later dormancy breaking and 5-15 days later budbreak. However, the present study was focused on the observatons from the IPG garden at Fana, because according to Wielgolaski et al. [4] the negative trend in spring temperatures was most pronounced in the coastal areas north and south of Bergen.
In order to confirm the conclusions from Nordli et al. [2] and Wielgolaski et al. [4] on a close relationship between climatic warming and earlier budbreak in tree species, the dates of budbreak (Julian days) were plotted against mean January-April temperature at Fana for the species and provenences listed in Table 1, where the numbers refer to the IPG classification system. The regression analysis is shown in Table 2. In Norway spruce (Picea abies) and beech (Fagus sylvatica) three provenances were investigated, hence these two species were chosen for further analysis, in contrast to the remaining tree species, where only one provenance was studies for each species.
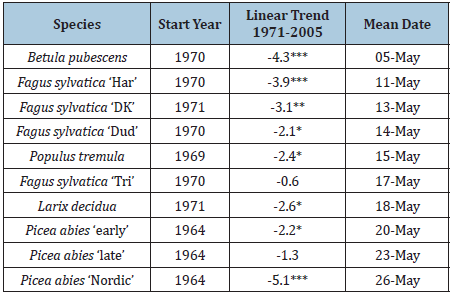
Table 2: Starting year, linear trend 1971-2005 and mean date of budbreak for Investigated species. Significance levels indicated by asterisks, ***P<0.01, **p<0.01, *p<0.05.
According to Table 1 the two beech provenances ‘DK’ and ‘Dud’ are intermediate, and in order to avoid unnecessary confusion, only the earliest (‘Har’) and latest (‘Tri’) provenances are included in our analysis. The date of budbreak is defined by the time when 50 of the buds are opened (first leaf visible). In Prunus avium, Ribes alpinum and Sorbus aucuparia also the date of flowering, as defined by 50% opening of flower buds, were recorded.
Results and Discussion
The mean January-April temperatures at Fana increased from about 1.5 to 3.0 °C during the observation period (Figures 3 & 4) Consequently, a significant trend against earlier budbreak was found during the period, and this change was closely correlated with increased temperature [2]. The temperature variation during the sample period 1964-2014, showing a significant negative trend in the onset of phenophases after 1980 caused by man-made climatic warming, and a corresponding significant increase in spring temperatures, are in strong contrast to the development during the period 1928-77 [4], showing decadal variations, probably caused by variations in solar activity, in addition to a weak climatic trend.
Provenance variations
(Table 1) In both investigated species, e.g., spruce (Picea abies) and beech (Fagus sylvatica), the budbreak occurred about 10 days earlier in beech than in spruce in the beginning of the observation period, while there was no difference late in the period (2014). Consequently, the shift in the phenophase occurrence seemed to be stronger in spruce than in beech, which may be partly related to the seasonal temperature variations (Figures 3-5).
Figure 3: Dates of budbreak (Julian days) during the sample period 1964-2014 in three Norway spruce (Picea abies) and two beech (Fagus sylvatica) provenances in the IPG garden at Fana. The lower diagram is showing the January-April mean temperature in the same period (°C).
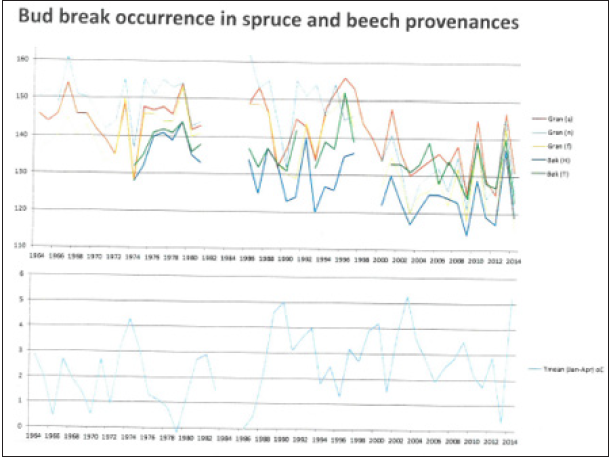
Figure 4: Dates of budbreak and flowering (Julian days) during the sample period 1964-2014 in Ribes alpinum, Sorbus aucuparia and Prunus avium in the IPG garden at Fana. The lower diagram is showing the January-April mean temperature in the same period (°C).

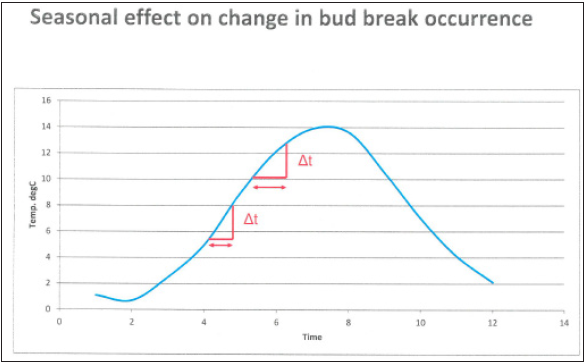
Figure 5: Diagram illustrating the effect of seasonal variations in temperature on the time interval corresponding to a certain temperature change, shown in red, at spring and summer conditions.
When comparing the three investigated spruce provenances, budbreak generally occurred earliest in the ‘early’ population from Germany, followed by the ‘late’ German population, and at last the ‘Nordic’ population from Norway (Figure 3), although these trees were from a much higher latitude than the ‘late’ population. The difference varied from 5 to 15 days. The most probably explanation is the high altitude of the mother trees of the ‘late’ population, as compared with the ‘Nordic’ relative. Early budbreak is not only found in northern provenances of tree species, but also in provenances from high altitudes [9,10]. However, Figure 3 shows, however, that by the end of the observation period the budbreak occurred almost at the same time in the ‘late’ and ‘Nordic’ spruce trees.
A similar comparison between two of the investigated beech provenances showed – as expected – that budbreak occurred 3-12 days earlier in the northern provenance ‘Har’ than in the southern relative ‘Tri’ (Table 1). The two remaining provenances (‘Dud’ and ‘DK’) were intermediate, although the ‘DK’ plants were from a more northern origin than ‘Har’, but from a 200meter lower altitude.
Comparison between date of budbreak and flowering
In three of the investigated species (Figure 4) the date of flowering (e.g., 50% opening of flower buds) were also recorded. Earliest budbreak and flowering occurred in Ribes alpinum, followed by Sorbus aucuparia and Prunus avium with about 35 days difference between Ribes and Prunus during the whole observation period. After the warm winters 2000-14 the budbreak date in Ribes alpinum dropped to below 100 Julian days, e.g., 10 April or earlier.
In Ribes alpinum the flowering occurred about 6 days later than budbreak in the beginning of the observation period, and this delay had increased to about 24 days by the end of the period (Figure 4). A similar trend was also found in Prunus avium, while the interval between budbreak and flowering remained constant at about 20 days in Sorbus aucuparia. As a consequence, flowering in Ribes arcticum and Prunus avium seemed to be less influenced than budbreak by climatic warming, probably because other factors than temperature, e.g., solar radiation also may influence on the development [2].
Comparison between species
Figure 6: Dates of budbreak (Julian days) during the sample period 1964-2014 in the IPG garden species at Fana mentioned in Table 1. The lower diagram is showing the January-April mean temperature in the same period (°C).
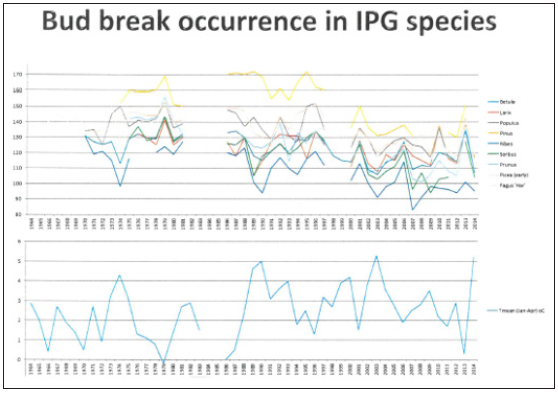
In Figure 6 the budbreak dates in all nine investigated species during the observation period are shown. The difference between the species with earliest budbreak (Ribes alpinum) and the corresponding late budbreak species (Pinus sylvestris) dropped from about 55 days to 30 days during the observation period (1964-2014). As a consequence, the change toward earlier budbreak appeared to be stronger in species with late phenophases like Pinus sylvestris than in species with early phenophases like Ribes alpinum and Betula pubescens. The change was about 35 days in Pinus but only about 15 days in Ribes and Betula. This may be caused partly by genetic differences but may also just be connected to the fact that more days are normally needed in late spring (May) to get the same temperature increase as in early spring (April), due to the sinoid shape of the annual mean temperature function (Figure 5).
Anyway, the observed climatic change and related earlier phenophases in many tree species and provenances during the last 50 years are expected to lead to extended seasonal growth, which again would be leading to better seed reproduction and tree line advance in the alpine and arctic areas throughout Fennoscandia, while a number of cold-adapted shrubs and herbs may suffer from the increased winter temperatures [9,11].
Conclusion
The results from the present analysis of the occurrence of spring phenophases in the IPG garden at Fana during the observation period 1964-2014 may be summarized as follows:
A close relationship was found between mean annual spring temperature (January-April) and the corresponding date of budbreak in all investigated species.
As a consequence, the dates of budbreak were occurring.
15-35 days earlier in 2014 than 40 years before.
The shift against earlier budbreak was stronger in tree species with late budbreak than in relatives with early budbreak.
Ecotypic variations were found between provenances of Picea abies and Fagus sylvatica, e.g. budbreak occurred earlier in northern and high-elevated provenances than in southern and low-elevated relatives.
Date of flowering in Prunus avium and Ribes alpinum was less influenced by climatic warming than budbreak occurrence, while in Sorbus aucuparia the dates of budbreak and flowering were equally influenced.
References
- Chmielewski FM (1996) The international phenological gardens across Europe. Present state and perspectives. Phenol Season 1: 19-23.
- Nordli Ø, Wielgolaski FE, Bakken AK, Hjeltnes SH, Måge F, et al. (2008) Regional trends for bud burst and flowering of woody plants in Norway as related to climate change. International Journal of Biometeorology 52: 625-639.
- Lauscher A, Lauscher F, Printz H (1955) Die phenologie norwegens, Teil I. Allgemeine Ü Skr Norske Vid-Akad Oslo. In: Mat Naturv Klasse no 1: 1-99.
- Wielgolaski FE, Nordli Ø, Karlsen SR, B O’Neill (2011) Plant phenological variation related to temperature in Norway during the period 1928-1977. Int J Biometeorol Springer 12: 819-830.
- Myking T, Heide OM (1995) Dormancy release and chilling requirements of buds of latitudinal ecotypes of Betula pendula and B. pubescens. Tree Physiology 15(11): 697-704.
- Skre O (2019) Northern tree lines as indicators of climate and land use changes. A literature review. Agrotechnology 8(2).
- Taulavuori K, Taulavuori E, Skre O, Nilsen J, Igeland B, et al. (2004) Dehardening of mountain birch (Betula pubescens ssp.czerepanovii) ecotypes at elevated winter temperatures. New Phytol 162(2): 427-436.
- Skre O, Taulavuori K, Taulavuori E, Nilsen J, Igeland B, et al. (2007) The impact of hardening and winter temperature for growth in mountain birch populations. Environmental and Experimental Botany 62(3): 254-266.
- Skre O, Nilsen J, Naess M, Igeland B, Taulavuori K, et al. (2005) Responses of temperature changes on survival and growth in mountain birch populations. In: Wielgolaski FE (Ed.), Herbivory and human impact in Nordic mountain birch forests. pp. 130-145.
- Ovaska JA, Nilsen J, Wielgolaski FE, Kauhanen H, Partanen R, et al. (2005) Phenology and performance of mountain birch provenances in transplant gardens; Latitudinal, altitudinal and oceanity-continentality gradients. In: Wielgolaski FE (Ed.), Plant ecology, herbivory and human impact in nordic mountain birch forests. Ecological Studies, Springer, Germany, 180: 99-115.
- Hofgaard A, Tømmervik H, Rees G, Hanssen F (2013) Latitudinal forest advance in northernmost Norway since the 20th J Biogeogr 40(5): 938-949.
© 2021 Oddvar Skre. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






