- Submissions

Full Text
Novel Research in Sciences
Establishing a Water-Chemical Regime and Increasing the Efficiency of Combustion of a Mixture of Fuel Oil and Gas in a de 25-14gm Boiler at the Kakand Distillery
Yusupaliev R, Shamsiev K, Azimova M and Musashayxova N*
Tashkent State Technical University named after Islam Karimov, Uzbekistan
*Corresponding author:Musashayxova N, Tashkent State Technical University named after Islam Karimov, Department of Power, Tashkent, Uzbekistan
Submission: February 16, 2021;Published: April 8, 2021
.jpg)
Volume7 Issue1April, 2021
Abstract
In the article, it is considered to establish the water-chemical regime on the DE 25/14 boiler, before starting alkaline washing with solutions of sodium hydroxide and sodium phosphate. The boiler washing procedure was carried out in five stages. After the end of flushing, the volume of flushing water was experimentally determined at various pressures. The article also describes how to establish the optimal mode of combustion of a mixture of gas and fuel oil in furnaces and performed heat engineering tests to determine the flow rate of ammonia when cleaning flue gases from sulfur oxide.
Keywords: Drum; Steam generator; Water treatment; Boiler and feed water; Salinity; Boiler cleaning; Static pressure; Substances; Turbine; Low- and high-pressure heaters; Deaerator; Ion exchange filters; Sodium; Hydrogen cation exchange filters
Introduction
To establish the water-chemical regime and determine the volume of purge water in the DE 25/14 boiler at various pressures, a pre-start alkaline flushing was carried out in accordance with the instructions with a mixture of 0.5% sodium hydroxide solution and 0.3% solution of trisodium phosphate Na3PO4 [1,2]. Prestarting alkaline flushing of the boiler is carried out to remove corrosion products and to form an oxide film of Fe3O4 on the metal surface in order to protect the metal from corrosion processes. Before alkaline washing, an external examination of the water-steam tract and its tightness were carried out, and the required amount of alkali and trisodium phosphate consumption for the preparation of their solutions was determined.
Procedure for pre-start alkaline flushing of the boiler:
a) preparation of the boiler for flushing.
b) preparation of solutions of the necessary reagents.
c) carrying out pre-start alkaline washing of the boiler.
d) 4-implementation of organizational work when starting up the boiler.
e) organization of the water chemistry regime and determination of the volume of
boiler blowdown water at various pressures.
Considering the water volume of the DE-25/14 boiler, which is 16.5m3, according to the calculation for the preparation of a 0.5% NaOH solution, 85kg of 100% NaOH and 50 kg of 100% Na3PO4 were pleased [3]. After preparing the working solution, the boiler was filled with feed water and kindling began.
Results and Discussion
During the firing up of the boiler, when checking the tightness of the water-steam path, it was necessary to replace the gaskets of the blowdown drain valve. The duration of each experiment at various pressures of 3, 6, 10 and 13ata was 7-8 hours. Between experiments, the boiler was periodically purged. The control of the prestarting alkalization regime of the boiler at such pressures (3.5, 10, 13ata) was carried out by determining the composition of the boiler water, its alkalinity and salt content [4]. After completing alkalization, the boiler was stopped for 15-20 hours to cool down, then the reagent solution was drained, and its internal inspection was performed to check the results of alkalization and the formation of an oxide film on the metal surfaces. Inspection showed that alkalization proceeded as instructed and the expected results were obtained. Then, having again filled the boiler with feed water, organizational work was carried out for commissioning and for establishing a water-chemical regime with determining the volume of blowdown water at various pressures.
The table shows reductions in boiler steam output depending on pressures (Table 1).
Table 1: Decrease in boiler steam output depending on steam pressure.
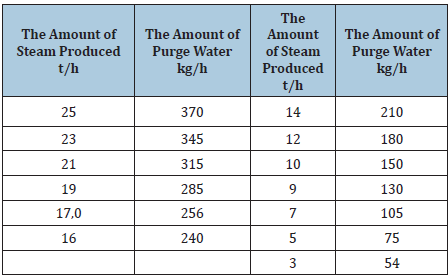
As you know, the efficiency of the boilers and the reduction of fuel consumption mainly depends on the establishment of the optimal mode of fuel combustion in the furnaces. In order to increase the efficiency and reduce the number of emissions in case of incomplete combustion of fuel, we performed heat engineering tests of the DE-25-14GM boiler when burning a mixture of fuel oil and gas (heat of combustion of fuel oil QPH = 10195 kcal/kg, natural gas QpH = 8247kcal/kg). Measurements and processing of test results were carried out in accordance with accepted methods. The frequency of the analysis with the performance of the boiler unit and the properties of the flue gases was 60 minutes. The operating mode of the boiler units in terms of such indicators as the steam pressure in the drum, the temperature of feed water, natural gas, fuel oil, exhaust gases and cold air in front of the burners was monitored using panel and local devices [5-7]. An electronic gas analyzer "KAME-MAU-9104" was used to measure the temperature of flue gases and analyze their composition at the sampling point. A sample of flue gases was taken from a sampling point located between the economizer and the boiler exhaust fan. Pressure, fuel and air temperatures were measured directly at the burner. In each experiment, readings of standard instruments were taken, the content of carbon oxides (CO, CO2) was recorded. The tests of the DE-25-14GM boiler were carried out in the load range of 9-22 t/h at the pressure in the boiler drum at the level of 0.5-0.8MPa (working pressure), the excess air ratio in front of the smoke exhauster a = 2.5-1.4. The exhaust gas temperature varied within 130-160 °C. Analysis of the test data shows that the boiler operates with increased excess air and gases, but the boiler operates stably in the tested load range. The test results of the DE-25-14GM boiler and the dependence of the technical and economic indicators of its operation on the excess air ratio are shown in (Table 2). The table shows that when burning in boilers, sweep away fuel oil and gas, the flue gases contain a large amount of carbon monoxide CO due to incomplete combustion fuel and in our opinion, this phenomenon is associated with the supply of heated air to the combustion chambers, which is not provided for by the boiler design [7,8]. Considering all of the above, in order to reduce heat losses with exhaust gases and reduce the amount of carbon monoxide in them, it is advisable to heat the air supplied to the furnace, for which we proposed it is necessary to install an air heater after the economizer.
Table 2: Test results of the DE-25-14GM boiler under various loads during the combustion of a mixture of fuel oil and natural gas.

When fuel oil is burned in the furnaces of steam generators, harmful gases are formed in very large quantities, such as oxides of sulfur, carbon and various organic carcinogenic substances. The amount of the formed toxic substances mainly depends on the following factors: rational organization of combustion processes, the content of components in fuels and excess air (a) required for fuel combustion [9-12]. In the process of removing flue gases, the process of sulfuric acid corrosion of the surfaces of the heat exchange equipment of the tail sections of the boiler is accelerated, and when they are scattered, the atmospheric air is polluted. The reason for the occurrence of sulfuric acid corrosion is a decrease in the temperature of the exhaust gases in the tail section of the steam generators and the acceleration of the formation of sulfuric acid. It should be noted that one of the methods to prevent corrosion of the tail section of the steam generator is to reduce the concentration of sulfur oxides in the exhaust gases.
Taking into account the intense interaction of ammonia and oxides of sulfur and carbon, we propose the injection of ammonia solution into the zones where sulfuric acid corrosion is intense. Ammonia is widely used in thermal power plants when organizing water regimes to prevent carbon dioxide corrosion in the feed water path. The reactions of binding oxides of sulfur and carbon proceed according to the following equations:
2NH40H+2S02+02 = 2NH4HS04
2NH40H+S03 = (NH4)2S04+H20
NH40H+C02 = NH4HO3
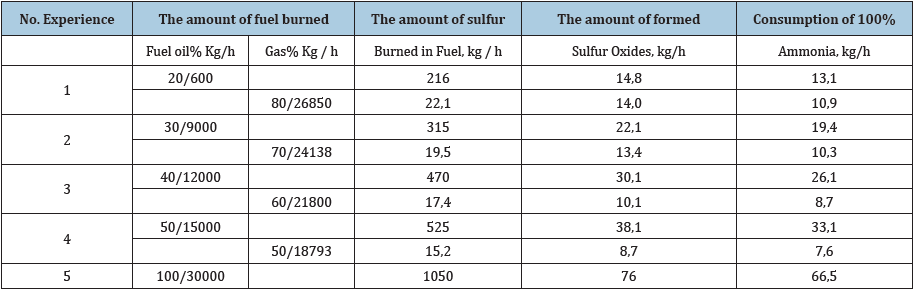
The calculation of the consumption of ammonia for the binding of sulfur oxides in steam boilers with the combined combustion of gas and fuel oil with an hourly consumption of fuel oil of 30,000kg and with a sulfur content of 3.5%. The calculation results are presented in (Table 3).
Table 3: Dependence of ammonia consumption on the amount of fuel burned.
Conclusion
As can be seen from the above data, an increase in the thermal fraction of fuel oil during co-combustion of fuel oil and gas increases the formation of sulfur oxides and, accordingly, increases the consumption of ammonia. It should be noted that, as a result of ammonia flue gases, simultaneously with the prevention of sulfur-oxygen corrosion in heat exchange equipment, the volume of harmful emissions into the atmosphere is also reduced.
References
- Abramov VI (2002) Improving the ecological safety of thermal power plants. M, Edn. MEI.
- Beloselsky BS (2003) Technology of fuel and energy oils. M, Edn. MEI.
- Yusupaliev RM, Usmonov NO (2016) Experimental electrocoagulation plant for preliminary purification of natural water for steam generation at TPPs. International Journal of Energy Saving Energy Audit Kharkov.
- Yusupaliev RM, Usmonov NO (2017) Liquid two-channel universal batcher for measuring the concentration of a reagent solution during water treatment. International Journal of Energy Safety and Energy Saving, Scientific-analytical and Educational-Methodical Journal Moscow.
- Yusupaliev RM, Musashaikhova NA (2018) Carrying out alkaline washing and adjusting the water-chemical regime of the DE-25/14 GM boiler. International VII Scientific and Technical Conference Saratov.
- Yusupaliev RM, Azimova MM (2019) Composition of natural waters of some sources of rivers of the Republic of Uzbekistan used in heat and power engineering and the results of experimental studies with preliminary and ion exchange water purification. International Scientific Seminar named after Yu.N. Rudenko 91st meeting, Tashkent city, Tashkent State Technical University. E3S Web of Cjnferencts 139 01083.
- Beloselsky BS, Solyakov VK (1998) Energetic fuel M. Energy.
- Reznikov MI, Lipov Yu M (1981) Steam boilers of thermal power plants. M Energoatomizdat.
- Khzmalyan DM, Kagan Ya A (2010) Combustion theory and furnace devices. M Energia.
- Shamsieva NK, Shamsiyev KS (2019) Intensification of the process of hydrodynamics and kinetics of drying dispersed materials in vortex dry camera. International Journal of Advanced Research in Science, Engineering and Technology 6(4): 8893-8897.
- Karimov R, Rasulov A, Shamsiyev K, Bekishev A, Kurbanova N, et al. (2020) Reliability indicators of stabilizing devices in the agriculture electrical supply system. IOP Conf Series: Materials Science and Engineering 883(1): 012142.
- Shamsiyev KS (2020) Issues of cotton cellulose and its properties as an object of technological processing. E3S Web of Conferences 216, RSES, Pp. 1-4.
© 2021 Musashayxova N. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






