- Submissions

Full Text
Novel Research in Sciences
Synthesis and Anti-inflammatory Activity of Newly Synthesized 1,7-Dimethyl-4H-Benzo [D] [1,3]-Oxazin-4-One and 3-Amino-1,7- Dimethyl-Quinazolin-4(3H)-One
Peter Osarodion O*
Department of Chemical Sciences, Nigeria
*Corresponding author: Peter Osarodion O, Department of Chemical Sciences, Nigeria.
Submission: February 22, 2021;Published: March 04, 2021
.jpg)
Volume6 Issue2March, 2021
Abstract
Background/Introduction: The synthetic studies of 4-quinazolinone derivatives have been presented due to the chemical and biological interest. 4-Quinazolinone derivatives possesses antibacterial, antifungal, amti-inflammatory, analgesic activities.
Aims and objectives: The objective of the present study was to synthesize these quinazolinone derivatives 3-Amino 7-methyl-1-methyl-4H-benzo[d]-[1,3]-oxazin-4-one and 3-Amino-7-methyl-2- methyl-3H-quinazolin-4-one and screened them for their anti-inflammatory activity.
Materials and methods: The condensation of 2-amino-methyl-5-methylbenzoate with acetic anhydride yielded the cyclic compound 1, 1,7-dimethyl-4H-benzo[d]-[1,3]-oxazin-4-one which further produce a novel 2,3-disubstituted-quinazolin-4 ones via the reaction with hydrazine hydrate. The compounds synthesized were unequivocally confirmed by means of Infrared, Nuclear Magnetic Resonance (1H and 13C), Gas Chromatography Mass Spectrophotometer and Elemental analysis. The synthesized compounds were screened and pharmacologically screen them for their anti-inflammatory activity.
Conclusion: Compound 2 showed a higher anti-inflammatory activity against compared to compound [1].
Keywords: 1,7-Dimethyl-4H-benzo[d]-[1,3]-oxazin-4-one and 3-Amino-2,7-Dimethyl-3H-quinazolin-4- one; 2-amino-methyl-5-methylbenzoate; Nucleophile; Anti-inflammatory activity
Material and Methods
General experimental procedure
All reagents and solvents were purchased from sigma-Aldrich, in Germany. Melting points were determined on a kofler hot stage apparatus and were uncorrected. IR spectra were recorded on a Buck scientific IR M500 instrument. The 1H and 13C NMR spectra were recorded in DMSO-d6 at 400MHz with HAZ VOLATILE V2. M Chemical shifts are reported in ppm relative to tetramethylsilane. Gas chromatography mass spectra were obtained on a Finingan MAT 44S mass spectrophotometer operating at 70eV. Elemental analysis was carried out with analytical. Thin layer chromatography (TLC) was used to monitor the reactions.
Synthesis of 7-methyl-1-methyl-4H-benzo [D] [1,3]-oxazin- 4-one (1)
This involved the condensation of Methyl-2-amino-7-methylbenzoate 2.11g(0.01mol) and 1.02g (0.01mol) acetic anhydride in 30ml ethanol medium were reacted. The reaction was heated under reflux with stirring using a magnetic stirrer until the reaction mixture showed no trace of starting material when the TLC was developed (2 hours). At the end of the reaction, work up was done. Ethanol was removed in vacuum and the crude mixture was poured into 50ml of ice water on a cold-water bath. The mixture was stirred for 30 minutes filtered and extracted into ethyl acetate and allowed to evaporate at room temperature to give solid products which were recrystallized from hexane or dichloromethane-hexane mixture. Yield was 2.01g (95%), mp: 148-150 oC (Figure 1).
Figure 1:
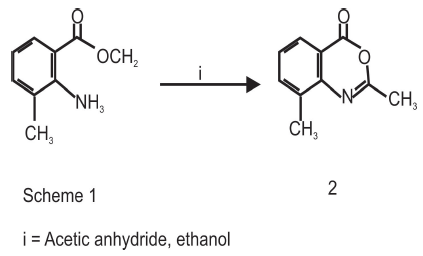
Synthesis of 3-amino-7-methyl-1-methyl quinazolin-4 (3H)-one. (2)
The condensation of equimolar amounts of 2-methyl-7-methyl- 4H-benzo [D] [1,3]-oxazine-4-one (1.06g, 0.005mol) and hydrazine hydrate (0.93g, 0.01mol) were added to 30ml boiling ethanol with stirring using a magnetic stirrer until the reaction mixture showed no trace of starting material when the TLC was developed (3 hours). At the end of the reaction, the reaction mixture was concentrated in vacuum under reduced pressure using rotary evaporator. The white precipitate formed was then filtered, washed three times with 20ml of distilled water [20mlx3]. The white crystals were dried and recrystallized from dimethylformamide (DMF) to give pure 3-amino-2-methylquinazolin-4 (3H)-one. Yield was 1.00g (94%) mp: 97-99 oC (Figure 2-4).
Figure 2:
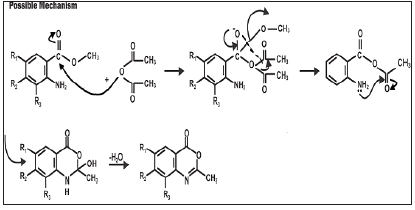
Where: R1 = H, R2 = H and R3 = CH3
Figure 3:

Figure 4:
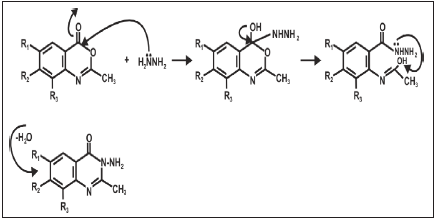
Where: R1 = H, R2 = H and R3 = CH3
Chemistry
The introduction of 2-amino substituent is a successful strategy to improve the chemical stability of benzoxazinone. Due to the pharmacological activities of 4(3H)-quinazolinone derivatives, 2,3-disubstituted derivative of quinazoline-4-one were synthesized via the interaction of the benzoxazinone derivative with nitrogen nucleophile with the aim of obtaining more pricise information about the course of the reaction and some interesting pharmaceutical compounds. The reaction of 4, 5-disubstituted derivatives of 2-amino-methyl-5-methylbenzoate and acetic anhydride yielded the cyclic compound 7-methyl-1-methyl-4H-benzo[d]-[1,3]-oxazin- 4-one. The reaction of this compound with hydrazine hydrate yielded 3-Amino-7-methyl-1-Methyl Quinazolin-4 (3H)-one.
Pharmacological evaluation
Anti-inflammatory activity: Anti-inflammatory activity will be measured using carrageenan-induced rat paw oedema assay [22,23]. Groups of 5 rats of both sexes (pregnant female excluded) were given a dose of an anti-inflammatory activity of synthesized compounds. After one hour 0.1ml, 1% Carrageenan suspension in 0.9% NaCl solution were injected into the sub-planter tissue of the right-hand paw. The linear paw circumference was measured at hourly interval for four hours [24]. Two groups of drugs treated rats and one control group were used each test day and the mean paw oedema value for the test group being compared with the mean value for the control group for that day. Anti-inflammatary activity [25] will be measured as the percentage reduction in oedema level where drug was present, relative to control. Indomethacin (10mg/ kg) was administered orally as reference drug, whereas 10% Tween 80 was uses as negative control.
Statistical analysis: All data were expressed as the mean + SEM, the student’s t-test was applied to determine the significance of the difference between the control group and the test compounds.
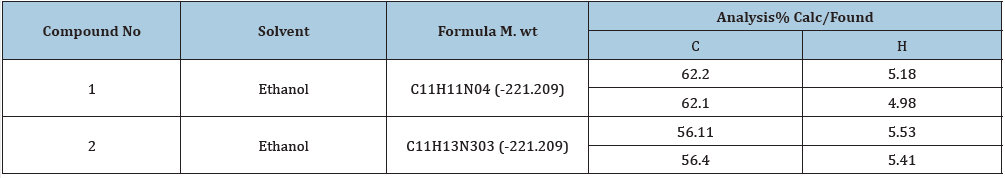
Elemental analysis: The compositions of the compounds are summarized in Table 1. The C and H contents (both theoretically calculated values and actual values) are indicated
Table 1:Characterization and physical data of synthesized compounds.
Result
Table 2:13C-NMR of synthesized compounds.
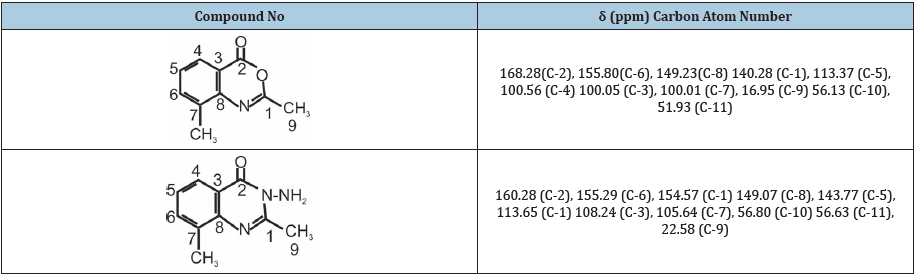
Table 3:13C-NMR of synthesized compounds.
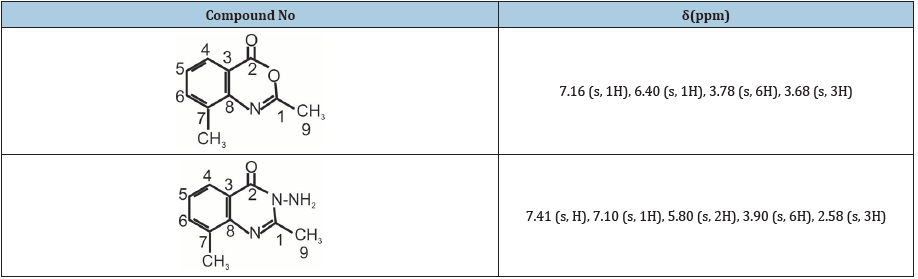
Table 4:Effect of the test compounds on acetic acid induced writhing in mice.

The in vivo analgesic activity of 7-chloro-2-methyl4[H]- benzo[d][1,3]-oxazin-4-one (1) and 3-amino-7- chloro-2-methylquinazolin- 4(3H)-one (2) was determined using mouse writhing assay and the results obtained are summarized in Table 1-4.
Characterization of 7-methyl-1-methyl-4H–benzo[d] [1,3]–oxazine–4–one (1)
1H NMR (400MHz, DMSO) δ 7.16 (s, 1H), 6.40 (s, 1H), 3.78 (s, 6H), 3.68 (s, 3H), 13(NMR (400MHz, DMSO) δ 168.28, 155.80, 149.23, 140.28, 113.37, 100.56, 100.05, 56.94, 56.94, 56.13, 51.93, 16.95: IR (KBr, cm-1) 3381, 3203, 3135, (NHz), 3018 (CH aromatic), 2951, 2871, 2718 (CH aliphatic), 1662 (C=0). Anal.Cal 1159 (c-0) for C11H11N04; C 62.20; H 5.18. Found: C 62.10, H 4.98.
Characterization of 3-amino-7-methyl 2-methyl quinazoline-4-(3H)-One (2)
1H NMR (400 MHz, DMSO) δ 7.41 (s, 1H), 7.10 (s, 1H), 5.80 (s, 2H), 3.90 (s, 6H), 2.58 (s, 3H), 13C NMR (400MHz, DMSO) δ 160.28, 155.29, 154.57, 149.07, 143.77, 113.65, 108.24, 105.64, 56.80, 56.63, 22.58, IR (KBr, cm-1) 3301 (NH2), 1622 (C=0), Anal. Cal. for C11H13N303; C 56.11, H 5.53; Found, C 56.40, H 5.41.
Discussion
The test investigated compounds exhibited significant antiinflammatory
activity when compared with the control test sample.
For the IR spectra, compound 1 were characterized by absence
of υ NH2and presence of υ C-O stretch in 1159cm-1 region of the
compound. Compound 2 was characterized by absence of υ C-O
and presence of υNH2 in 3284cm-1and 3194cm-1 region of the
compound. Among the prepared products, 3-Amino-7-methyl-
2-methyl-3H-quinazolin-4-one (2) was found to exhibits the
most potent in vitro anti-inflammatory activity. The compounds
synthesized exhibited promising anti-inflammatory drug.
Compound 1 has Anti-inflammatory activities of 42.55% and
55.54% at 20mg/kg and 40mg/kg respectively while Compound
2 has Anti-inflammatory activities of 58.38% and 73.56%
respectively. Compound 2 showed the highest activity at 40mg/
kg compared to the other compound 1, acetylsalicylic acid and
indomethacin. It may be that the substitution of amino group at position three increases the activity. These compounds synthesized
have a higher activity than acetylsalicyclic acid, which is a standard
anti-inflammatory drug.
The acetic acid induced abdominal constriction method is
widely used for the evaluation of peripheral antinociceptive activity
[26]. It is very sensitive and able to detect antinociceptive effects
of compounds at dose levels that may appear inactive in other
methods like the tail-flick test [26,27]. Local peritoneal receptors
are postulated to be partly involved in the abdominal constriction
response [28]. The method has been associated with proteinoids in
general, e.g., increased levels of PGE2 and PGE2a inter peritoneal
fluids [29], as well as lipoxygenase products by some researchers
[30,31]. Indomethacin (10mg/kg) was administered orally as
reference drug while 10% olive oil was used as negative.
Conclusion
Compound 1 has Anti-inflammatory activities of 74.67% and 42.55% at 20 mg/kg and 54.45% at 40 mg/kg dose levels, while compound 2 has Anti-inflammatory activities of 58.38% and 73.56% at 20 mg/kg and 40mg/kg dose levels respectively. The compounds have high Anti-inflammatory activities. Compound 2 has a higher Anti-inflammatory activities compared to compound 1 and also has a higher Anti-inflammatory activity compared to Indomethacin, a standard Anti-inflammatory drug. It is therefore concluded that compound 2 could be a potential anti-inflammatory agent and a tool in pharmaceutical drug delivery.
Author’s Declaration
The author hereby declares that the work presented in this article is original and that any liability for claims relating to the content of this article will be borne by them.
Acknowledgement
The author appreciates the assistance of the Department of Pharmaceutical Pharmacology at the University of Benin who supplied the Swiss mice and Ethic approval of animal use from Ethics committee of the Faculty of pharmacy, University of Benin, Benin City Nigeria who gave the Ethic approval of animal use.
References
- Chandregowda V, Kush AK, Reddy CG (2009) Synthesis and in vitro antitumor activities of novel 4-anilinoquinazoline derivatives. Eur J Med Chem 44(7): 3046-3055.
- Al-Rashood ST, Aboldahab IA, Nagi MN, Abouzeid LA, Abdel Aziz AA, et al. (2006) Synthesis, dihydrofolate reductase inhibition, antitumor testing, and molecular modeling study of some new 4(3H)-quinazolinone analogs. Bioorg Med Chem 14(24): 8608-8621.
- Vasdev N, Dorff PN, Gibbs AR, Nandanan E, Reid LM, et al. (2005) Synthesis of 6-acrylamido-4-(2-[18F] fluoroanilino) quinazoline: A prospective irreversible EGFR binding probe. J Lablelled Compd Rad 48: 109-115.
- Wakeling AE, Guy SP, Woodburn JR, Ashton SE, Curry BJ, et al. (2002) ZD1839 (Iressa): An orally active inhibitor of epidermal growth factor signaling with potential for cancer therapy. Cancer Res 62: 5749-5754.
- Alagarsamy V, Solomon VR, Dhanabal K (2007) Synthesis and pharmacological evaluation of some 3-phenyl-2-substituted-3H -quinazolin-4-one as analgesic, anti-inflammatory agents. Bioorg Med Chem 15: 235-241.
- Baba A, Kawamura N, Makino H, Ohta Y, Taketomi S, et al. (1996) Studies on disease-modifying antirheumatic drugs: synthesis of novel quinoline and quinazoline derivatives and their anti-inflammatory effect1. J Med Chem 39(26): 5176-5182.
- Rohini R, Reddy MP, Shanker K, Hu A, Ravinder V (2010) Antimicrobial study of newly synthesized 6-substituted indolo[1,2-c]quinazolines. Eur J Med Chem 45(3): 1200-1205.
- Antipenko L, Karpenko A, Kovalenko S, Katsev A, Porokhnyavets KE, et al. (2009) Synthesis of new 2-thio-[1,2,4]triazolo[1,5-c]quinazoline derivatives and its antimicrobial activity. Chem Pharm Bull 57(6): 580-585.
- Jatav V, Kashaw KS, Mishra P, Gupta V (2008) Synthesis and antimicrobial activity of some new 3-[5-(4-substituted) phenyl-1,3,4-oxadiazole-2yl]-2-styrylquinazoline-4(3H)-ones. Med Chem Res 17: 205-211.
- Aly AA (2003) Synthesis of novel quinazoline derivatives as antimicrobial agents. Chin J Chem 21(3): 339-346.
- Li H, Huang R, Qiu D, Yang Z, Liu X, et al. (1998) Synthesis and bioactivity of 4-quinazoline oxime ethers. Prog Nat Sci 8: 359-365.
- Chandrika PM, Yakaiah T, Narsaiah B, Sridhar V, Venugopal G, et al. (2009) Synthesis leading to novel 2,4,6-trisubstituted quinazoline derivatives, their antibacterial and cytotoxic activity against THP-1, HL-60 and A375 cell lines. Indian J Chem 48B: 840-847.
- Paneersalvam P, Raj T, Ishar PSM, Singh B, Sharma V, et al. (2010) Anticonvulsant activity of Schiff bases of 3-amino-6,8-dibromo-2-phenyl-quinazolin-4(3H)-ones. Indian J Pharm Sci 72(3): 375-378.
- Nandy P, Vishalakshi MT, Bhat AR (2006) Synthesis and antitubercular activity of Mannich bases of 2-methyl-3H-quinazolin-4-ones. Indian J Heterocycl Chem 15(3): 293-294.
- Saravanan G, Alagarsamy V, Prakash CR (2010) Synthesis and evaluation of antioxidant activities of novel quinazoline derivatives. Int J Pharm Pharm Sci 2: 83-86.
- Lakhan R, Singh OP, Singh-J RL (1987) Studies on 4 (3H)-quinazolinone derivatives as anti-malarials. J Indian Chem Soc 64: 316-318.
- Hess HJ, Cronin TH, Scriabine A (1968) Antihypertensive 2-amino-4(3H)-quinazolinones. J Med Chem 11(1): 130-136.
- Sasmal S, Balaji G, Reddy KHR, Balasubrahmanyam D, Srinivas G, et al. (2012) Design and optimization of quinazoline derivatives as melanin concentrating hormone receptor 1 (MCHR1) antagonists. Bioorg Med Chem Lett 22(9): 3157-3162.
- Alvarado M, Barceló M, Carro L, Masaguer CF, Raviña E (2006) Synthesis and biological evaluation of new quinazoline and cinnoline derivatives as potential atypical antipsychotics. Chem Biodivers 3(1): 106-117.
- Malamas MS, Millen J (1991) Quinazolineacetic acids and related analogs as aldose reductase inhibitors. J Med Chem 34(4): 1492-1503.
- Ünlü S, Önkol T, Dündar Y, Ökçelik B, Küpeli E, et al. (2003) Synthesis and analgesic and anti-inflammatory activity of some new (6-Acyl-2-benzoxazolinoneand 6-Acyl-2-benzothiazolinone derivatives with acetic acid and propanoic acid residues. Arch Pharm Pharm Med Chem 336(8): 353-361.
- Writer CA, Risley EA, Nuss GW (1962) Carrageenan-induced Oedema in the hind paw of rat as an assay for anti-inflammatory activity. Procsoc Exp Biol Ther 111(3): 544-547.
- Adeyemi CO, Okpo SO, Ogunti CO (2002) Analgesic and anti-inflammatory effect of the aqueous extract of leaves of Persea America Mill (Lauraceae). Fitoterapia 73(5): 375-380.
- Bamgbose SOA, Noamesi BK (1981) Studies on cryptolapine Inhibition of carrageenan inducedoedema by cryplolepine. Planta Med 41(4): 392-396.
- Duffy JC, Dearden JC, Rostron C (2001) Design, synthesis and biological testing of a novel series of anti-inflammatory drugs J Pharm Pharmacol 53(1): 1505-1514.
- Gene RM, Segura L, Adzet T, Marin E, Inglesias J (2018) Heterothecai nuloides: Anti-inflammatory and analgesic effect. J Ethnopharmacol 60(2): 157-162.
- Koster R, Anderson M, Beer EJD (1959) Acetic acid for analgesic screening. Fed Proc 18: 412.
- Collier HOJ, Dinneen LG, Johnson CA, Schneider C (1968) The abdominal constriction response and its suppression by analgesic drugs in the mouse. British Journal of Pharmacology 32(2): 295-310.
- Bentley GA, Newton SH, Starr J (1981) Evidence for action of morphine and enkephalins on sensory nerve ending in the mouse peritoneum. British Journal of Pharmacology 73(2): 325-332.
- Bentley KW (1983) The isoquinolinalkaliods. In: Comprehensive Medicinal Chemistry, Pergamon Press, London, UK.
- Derardt R, Jongney S, Delvalcee F, Falhout M (1980) Release of prostaglandins E and F in an algogenic reaction and its inhibition. European Journal of Pharmacology 61(1): 17-24.
© 2021 Peter Osarodion O. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






