- Submissions

Full Text
Novel Research in Sciences
A Qualitative and Quantitative Review on Muscle Mass and Strength in Elderly People
Singh VK, Pattanaik S, Khan U, Jena T, and Upadhyay AK*
School of Biotechnology, Odisha
*Corresponding author: Upadhyay AK, School of Biotechnology, Odisha
Submission: Janaury 05, 2021;Published: February 08, 2021
.jpg)
Volume5 Issue4February, 2021
Abstract
Aging and increased debility are treated as medicative, socialistic as well as economic problems. At the age of about 45, muscle robustness/strength are meant to be well conserved but after the specified age performance reduces about 5-6% per decade. Nowadays, as aging comes earlier, it is important to raise resistance power in elderly people, so that they can do their to do list easily. In search of quality life, it is also important to maintain the storage of muscle protein and amino acids as we age. Observations led in model organisms exhibit that sarcopenia i.e., ameliorative and summed up loss of muscle mass and quality is carried out by fusion of muscle tissues foreign (extrinsic) and innate (intrinsic) factors and this change is very surprising from quick decay of muscles saw during disuse and fasting. As aging occurs, body composition goes into several changes which result in many metabolic disorders like hypertension, type-2 diabetes, insulin resistance and hyperlipidemia (means blood has too many fats or lipids) which causes increased cardiovascular death. Decrease in myosin heavy chain and mitochondrial proteins as a result of decreased synthesis of muscle proteins, occurs with age.
Introduction
The advancement in fields of health and nutrition is leading to an increase in lifespan, and thus demonstration of an ageing probability. Ageing population leads to many incurable diseases like diabetes, cardiovascular disease, cancers and even arthritis as a result of a sequential aging population [1]. In spite of the fact that it isn’t acknowledged as a clinically diagnosable thing because of an absence of agreement premise, muscle mass decays with propelling age in a cycle named sarcopenia, it has also been reported that people aged 75 years and greater lost their muscle at the rate of approx. 0.64-0.7% per year in women and around 0.8-0.98 % in men, additionally they also concluded that a person of age around 75 years is mostly targeted by Sarcopenia [2].
For diagnosing sarcopenia, the use of the presence of both low function (strength and execution) and mass of muscle has been specified by EWGSOP (European Working Group on Sarcopenia in Older People). EWGSOP antagonistically applies these attributes in addition characterizing the reasonable stages as ‘pre sarcopenia’, ‘sarcopenia’ and ‘extreme sarcopenia’. Reduced muscle mass without effect on muscle mass or physical activity is defined by the ‘presarcopenia’ process. Just strategies that measure muscle mass precisely, in contrast with standard populaces can characterize this cycle. Both low mass and capacity of muscle is portrayed by the sarcopenia cycle. The stage which is portrayed when every one of the three prerequisites i.e., low muscle mass, low muscle quality and low actual execution are met is extreme sarcopenia [3]. Glycolytic type IIb muscle filaments are more modest in both sarcopenia and cancer cachexia and are thus lost, while oxidative type I strands are regularly lost in stout people. Sarcopenia is corresponded with a few livings being wide changes in relation to morphological changes in the muscle, comprising an expansion for the measure of adipose tissue [4].
Metabolic changes in ageing were identified as a contributory factor to sarcopenia by Evans [5]. The impacts of muscle squandering and exhaustion offers ascend to different physical and psycho-social effects for example unfit to autonomously perform exercises of everyday life, shortcoming and more serious danger of falls, loss of free living and related burdensome side effects/social estrangement, absence of physical movement, higher danger of creating incurable sicknesses and higher danger of all-cause mortality [6]. The factors interact with lack of physical activity, decreased excretion of hormones, dietary deficiencies, and likely chronic inflammation, leading to a dynamic change in the structure of the body [7]. Other aging-related causes are systemic. There are enzymatic contrasts in the creation of energy with age; anaerobic compounds/enzymes will in general stay predictable with age, while the creation of aerobic/vigorous energy diminishes with age [8].
Sarcopenia is a long-term process in which, every year after maturity, some muscle mass is lost, and this cycle is clear in people more established than 60 years. Thus, muscle mass and robustness are independently controlled and muscle quality during maturing is a superior indicator of mortality. The age-related decrease of muscle quality can along these lines not be deciphered completely by the loss of bulk: in matured creatures, both muscle ‘amount’ and ‘quality’ decline. The entirety of this analysis will concentrate on the basic concept of ageing, mechanism of muscle mass behind it, atrophy & loss of muscle fibre & basic knowledge of metabolic consequences & potential interventions to minimize ageing [10].
Concept of Muscle Mass and Aging
As we grow older our muscle tissues undergo modifications, mostly related to loss of muscle mass and power. Before the age of 30, muscles tend to grow larger and stronger. However, eventually, in our 30s, we begin to lose bulk and muscle performances. The reason is age-associated sarcopenia. Muscle strength determines the physical performance which gait speed, in old, aged people [11]. Muscle mass is linearly correlated with muscle strength. The relationship between physical performance and muscle mass is the same as the relation between muscle strength and physical performance [12].
Sarcopenia isn’t always a disease rather refers particularly to the usual, involuntary decline in lean body mass that happens with age. At first, it was meant to decrease in lean mass and moreover it was also referred to rapid decrease in both muscle performance and size [13]. Sarcopenia brings about lop-sidedness between signals for muscle cell development and it additionally prompts for teardown. This leads to “Anabolism” which is the cell growth process and “catabolism” which is the cell teardown process [14]. Early symptoms of sarcopenia consist of feeling physically more fragile throughout the long term and having a larger number of issues than steady lifting familiar things. If a person easily becomes more exhausted, walks slowly and is less active then these symptoms might be showing decreased strength in a person. Dropping weight without attempting likewise can be a sign of sarcopenia [15].
Approx. 3-8 % muscle mass are meant to decrease per decade after the age of 30, but when we reach the age of 60 or greater, rapid decline in muscle mass is seen [16]. Sarcopenia leads to functional dependence and disability as it expands the danger of falls and weakness to injury. When there is loss in muscle mass then there is gain in fat mass and therefore changes the body structure which leads to an increase in insulin resistance at the old age people [17]. Moreover, when there is a decrease in bone density then joint stiffness increases, and there may be a small reduction in stature (kyphosis). These types of modifications have probable implications for several conditions, consisting of type 2 diabetes, weight problems, heart sickness, and osteoporosis [18].
The etiology of sarcopenia is multifactorial and complex. Decreased in physical activity level, declining androgen fixations, explicit wholesome lacks, persistent aggravation, insulin resistance are the main factor that results in sarcopenia and cachexia may likewise add to sarcopenia. Diagnosis of sarcopenia has to be taken into consideration at all old age sufferers who have decreases in actual capacity, quality and wellbeing. Sarcopenia must be taken into consideration for patients who are confined to bed by sickness or old age, experience issues in standing up from a seat, or who have a deliberate walk speed <1.0 m · s-1 [19].
Eating enough calories and top-notch protein can hinder the pace of muscle waste. Omega-3 and creatine enhancements may likewise help battle sarcopenia [20]. The most effective way to prevent and reverse sarcopenia is exercising. Indeed, even straightforward activities like strolling can slow your pace of muscle mass loss and being active is the most important thing to prevent muscle loss [21].
Mechanism Behind Skeletal Muscle Aging-Case Study from Drosophila and Mammalian Models
Drosophila as a rising version of muscle getting old (muscle aging)
The intrinsic and extrinsic modifications that usually control muscle ageing in humans were also observed in rodents, suggesting that rodents are immediate replica of human sarcopenia. However, as regularly performed in invertebrates, the high costs associated with storing rodents over their lifetime, the modest number of creatures that can be considered per condition, and the powerlessness to direct enormous scope in vitro hereditary screens demonstrate that reviews in basic model life forms could give significant extra knowledge into the etiology of sarcopenia. Genetic studies in the fruit fly (Drosophila melanogaster) have enhanced our interpretation of the ageing-regulating evolutionary retained signaling pathways, finding many mutants with extended or shortened life spans [22]. The association and digestion of skeletal muscle strands in Drosophila melanogaster is near that of warmblooded creatures. Imperfections in flight, climbing and velocity are progressively observable during the transitory life expectancy of natural fruit flies, i.e., roughly 2-3 months. Drosophila ‘s decrease in muscle mass most likely represents a reduced muscle strength; in adult flies, neither an age-associated reduction in absolute bulk nor loss of muscle atrophy/decay or different types of wastage have been recognized [23].
Studies are needed to confirm if there is a gradual decline in muscle mass during Drosophila ageing or if endocrine changes affect Drosophila muscle ageing like they do in mammals. The accessibility of a hereditary variety structure that permits genomewide, muscle-explicit examination of quality capacity during the maturing cycle and because of drug, nourishing and natural intercessions is supported by studies in Drosophila. Drosophila can give significant experiences into the inherent instruments controlling age-related muscle brokenness, despite its current lack of studies on mentioned qualities of sarcopenia and likely disparities with mammalian framework [10].
Studies are needed to confirm if there is a gradual decline in muscle mass during Drosophila ageing or if endocrine changes affect Drosophila muscle ageing like they do in mammals. The accessibility of a hereditary variety structure that permits genomewide, muscle-explicit examination of quality capacity during the maturing cycle and because of drug, nourishing and natural intercessions is supported by studies in Drosophila. Drosophila can give significant experiences into the inherent instruments controlling age-related muscle brokenness, despite its current lack of studies on mentioned qualities of sarcopenia and likely disparities with mammalian framework [10].
Foreign factors influencing sarcopenia
Sarcopenia evolves by different pathways in humans. Social and environmental conditions include extrinsic factors, such as: lighting; rough surfaces; carpets and rugs; various items on the ground; handrail-free stairs and unbound animals. In combination with age and the amount of risk factors present, the probability of falling increases [24]. A meta-examination of the impact of external factors on the likelihood of falls among the older people stated that residential approaches should be part of fall prevention planning strategies [25]. For example, in addition to showing neuronal loss and diminished regenerative ability, elderly individuals usually have lower supplement consumption, actual movement and anabolic hormone levels than young individuals. Sarcopenia often raises the susceptibility of the elderly to numerous extremely catabolic disorders, such as cancer, heart failure (chronic obstructive pulmonary disease), and spinal injury [10]. Pharmaceutical drugs for malignancy and any age-associated illnesses, in particular, also cause muscle pain as a side effect, making sarcopenia worse [10]. A portion of the endocrine changes and outer variables affecting the advancement of sarcopenia in warm blooded creatures and Drosophila are portrayed.
Endocrine regulation of sarcopenia
Skeletal muscle is known to atrophy in the middle or old age, the mechanism of muscle mass reduction is not understood. For regulating muscle growth, several studies found growth factors and cytokines play a major role [26]. During aging, changes in the endocrine environment led to succession and limited reversibility to sarcopenia. Doing so during aging, production of IGF-1 and other anabolic cytokines in tissues of muscle is inhibited resulting in reduced synthesis of mitochondrial proteins and myofibrillar (also known as muscle fibril) with increasing age [27]. Muscle-particular IGF-1 overexpression constricts the age-associated decrease of muscular tissues [28]. IGF-1 circulating level steadily rejected from early adulthood into progressive old age [29]. Insulin IGF-1 has been proven to aid cancers and shortens lifespan; however, long term management of IGF-1 probably won’t be suitable therapeutics of sarcopenia and cachexia [30]. More youthful muscles are more receptive to catabolic and anabolic stimuli than are matured muscles. In young age, protein synthesis in human muscles is normally stimulated by ingesting amino acids and by doing exercises, but not effective doing this at elderly stage, this event introduces a term “anabolic resistance” which may result in sarcopenia [31]. Glucocorticoids (a catabolic hormone synthesized in the adrenal cortex) inhibit protein synthesis and enhance proteolysis which affect muscle protein turnover (MPT) [32]. In response to glucocorticoids, protein degradation is increased, which has only been noticed in starved rats. This indicates that, in catabolic conditions, glucocorticoids can be a prime proteolytic pathway in response to acute stress [33]. They (glucocorticoids) help in inducing MuRF-1 and atrogin-1 (both are the key molecules involved in muscle atrophy) resulting ubiquitin proteasome system (UPS) to accelerate protein turnover [23]. Many studies have conflicting data regarding this, so cell affectability to catabolic and anabolic improvements in maturing and physiological impacts to these diminished affectabilities for unfamiliar components stay muddled [10].
Innate (intrinsic) malfunctioning causing loss of muscle function during aging
A numerous age associated innate malfunctioning/defects are detected in mammalian muscles and skeletal muscles of drosophila and other insects which decreases functional ability together with extrinsic factors [34].
Table 1: The common old age-related intrinsic defects/changes are noted in mammalian and Drosophila.
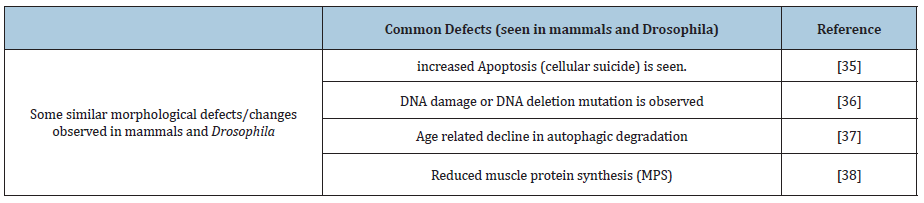
Some of the common old age-related intrinsic defects/changes are noted in mammalian and Drosophila (Table 1).
These similar defects in both Drosophila and mammals at old age suggest similarity in mechanism resulting in muscle functional decay at cellular level [10].
Variation in sarcomeres during aging
Sarcomere is the simple repeating of the basic rod-like unit of a muscle cell which is called Myofibrils; these filaments are organized into repeated subunits along the length of the myofibril in skeletal muscles. The sliding filament theory explains how muscles contract to produce force, the actin and myosin filaments within the sarcomeres of muscle fibres bind to create cross-bridges and slide past one another, creating a contraction, these cross-bridges are formed and the subsequent contraction of muscle [10].
In human beings and mice, age-associated structural and functional changes in sarcomeric proteins explains the decline in force generation which happens when a person grows old [35-39]. Research with permeabilized fibers and in vitro motility assays certainly highlight age-related intrinsic defects in the function of contractile proteins during old age, which includes reduction in actomyosin ATPase activity [40]. Study revealed that myosin (a superfamily of motor proteins) taken from young muscles have higher ability to move actin than the myosin taken from aged rodents and homosapiens [41].
In Drosophila, it has been reported that the sarcomere goes into several variations, affecting the consumption and functions of Myofibrillar protein [42]. It also includes increased disorganization and decreased length of the sarcomeres [43]. In contrast to Drosophila, mammalian models (mice) have numerous transcription factors (which includes serum response factor (SRF) and the autophagy adaptor proteins p62 (SQSTM1)) and NBR1(protein coding gene) localize to sarcomeres [44] probably to permit those factors to respond to modifications in contractile activity. Similarly, to influence muscle contraction, the age-associated disuse of sarcomeres may trigger changes inside the localization, it may also damage activity of sarcomere-related signaling factors which in turn resulting into some possible variations in cellular compartments [10].
Muscle Fibre Atrophy in Humans
Muscle Protein Synthesis (MPS) and Muscle Protein Breakdown (MPB) in human aging as an evidence of muscle fibre atrophy in humans
Muscle mass is regulated via the dynamic balance between MPS and MPB, with the major environmental effects on those methods being food consumption and physical interest. These regulations involve proteostasis also called as protein metabolism strictly happening at macroscopic level [45]. The intake of nutritional/ dietary protein impacts MPS by using the stimulation of MPS [46]. These anabolic responses are dose-based and saturable; at a maximal stimulus, rates of MPS increase ~200–300% for a duration of ~2 h following ~20 g protein [47]. Study revealed that excessive gain in MPS in response to AA infusion or following the proteostasis, this situation/condition is termed as anabolic resistance [48], they also concluded, the two factors which are responsible for the regulation of MPS i.e., postabsorptive and postprandial, are decreased under different muscle atrophy disuse conditions. The major chronic level reduction in MPS will result in skeletal muscle atrophy during disuse.
At the same time insulin suppresses MPB (mediated by using insulinogenic AA/or carbohydrate. Reflecting this, the anti-catabolic outcomes of insulin upon MPB was not recapitulated through AA infusions when insulin concentration had been clamped at 5μU. ml-1 post absorptive. Instead, insulin concentrations of simply 15 IU/ml (three × post absorptive) are enough to maximally suppress MPB [49]. This anti-catabolic effect of insulin acting on MPB become confirmed in a current systematic evaluate and meta-analysis of 44 human research, which concluded insulin did no longer notably have an effect on MPS however has an important role in lowering MPB [50]. Consequently, even as EAA’s stimulate MPS, insulin suppresses MPB (and stimulates muscle glucose uptake). On the basis, EAA and insulin are so critical in retaining muscle metabolic homeostasis, failure of these mechanisms unavoidably leads to skeletal muscle atrophy and IR [51]. This act to fill up muscle protein misplaced in the course of catabolism in the fasted state [52] e.g., due to efflux of muscle AA to assist hepatic gluconeogenesis. It’s been acknowledged for over 30 years that AA represent the primary nutrient driver behind feeding induced increases in MPS with this stimulation almost completely pushed by means of the important AA’s (EAA), mainly leucine [53]. Thus, uptake of leucine in excessive amounts (greater than that of defined saturable dose) may lead to unlikely increase in stimulation and duration of MPS.
Quantification of MPS and MPB in humans
In order to measure whole body muscle protein turnover (MPT) in humans to elaborate the effect of aging on skeletal muscle homeostasis, several isotopically labelled amino acids (AA; using 2H, 13C, 15 N, and 18O) tracers have been used to quantify the MPS [54]. Substantially, biopsy muscle sampling, extracting the tracer bound product (a product), typically the tracer in the plasma, into and out of tissues and proteins are used to determine Muscle Protein Synthesis (MPS) [45]. Comparing quantification of MPS, MPB is generally evaluated by using tracers across the organs, limbs and tissues. This is achieved at steady state by tracking the concentration of tracers in tissue arteriovenous. An elevation in MPB was seen when AA are released in muscle resulting into an increased venous dilution of tracer, also an elevation in MPB is termed as rate of appearance (Ra). They also coined the term net protein balance (NB) which is determined by the result of the balance between MPS and MPB at a steady state. By applying the measurement of Ra (rate of appearance) and NB (net protein balance), rate of disappearance (MPS) can be evaluated [53].
Mechanism of molecular regulation muscle protein turnover (MPT) and atrophy in humans
Muscle protein synthesis and its degradation is mainly governed by hormonal and some nutritional factors [55], by acting on specific receptors and effectors activates the translational initiation of protein synthesis [56]. Insulin, IGF-1(Insulin like Growth Factor-1), GH and testosterone are the basic hormones acting as a major effector for protein metabolism in the body [57]. In this regard [58] anticipated that anabolic hormone intervention within the variation of MPS after resistance exercise is more likely to be “chasing a hormonal ghost”. Thus, different local intramuscular mechanisms seem to screen the intense impact of the MPS reaction after resistance exercising.
The most abundant muscle AA mainly leucine and glutamine [59] act on different kinases to activate the stimulation of the initiation of translational synthesis of protein. An outstanding signaling pathway that controls the regulatory technique of protein synthesis entails the phosphorylation state of numerous regulatory proteins (AMPK, mTORC1, S6K1, MAPK) leading to improved myofibrillar protein synthesis after resistance exercising training and regulatory oxidative enzymes for the duration of persistence training [56]. Signaling thru mTORC1(mammalian or mechanistic target of rapamycin complex 1 an associated protein) is modulated via adjustments in activation of a couple of upstream pathways, a lot of which converge on the tuberous sclerosis complex (TSC) proteins TSC1 and TSC2 [60]. Also, a signal transduction network hub involved in the regulatory translation of mRNA [61]. Together these tuberous sclerosis complexes (TSC) 1 and 2 function to promote hydrolysis of GTP by the help of small GTPase (family of hydrolase enzyme) referred to as RHEB (ras homolog enrichment in brain) (Inoki et al. 2003). As Rheb is linked with GTP, it activates the mTORC1, whereas Rheb GDP does not activate the mTORC1 [62]. Thus, mTORC1 signaling is activated by the hormone such as insulin, IGF-1, testosterone and GH, also AA mainly leucine helps to stimulate mTORC1 signaling [63]. Mitogenic, exercise and nutrient- induced stimulation of protein synthesis in skeletal muscle is mediated by mTORC1 signaling. Although postabsorptive signaling through mTORC1 does now not appear to be attenuated in human disuse atrophy [64], mTORC1 signaling to p70S6K1 is repressed underneath postabsorptive situations in animal models of hindlimb immobilization. Mechanism for attenuated mTORC1 signaling is unknown, but researchers said that it may be a consequence of upregulated expression of mTORC1 such as regulated in DNA damage and development of 1 and 2 (REDDI1/2) proteins [65]. Few studies on miRNA also found that persistence/ endurance training down regulates numerous miRNAs focused on DNA sequences concerned in muscle differentiation [66]. Adding to it [67] concluded that numerous myomiRs (special class of miRNAs that regulate the gene expression in muscles) are down regulated at the end of the training period and in between the two months of cessation.
Muscle Fibre Loss in Human
Quantification of muscle fibre number (hypoplasia) in older humans
Beyond muscle atrophy, the second, and probable inter-related mechanism of complete muscle atrophy is that of muscle fibre loss (hypoplasia). The measurement of gold-standard is direct anatomical estimates acquired from cadaveric studies, despite the fact that for apparent motives those researches are rare. Some of the first human research of this nature came from Lexell who compared facts from 12 cross-sections of autopsied vastus lateralis (VL) of ∼30 and ∼72-year antique men [68]. The suggested general muscle size of the VL becomes 18% smaller in the old-aged people. The difference in general muscle size to be accounted for showed a marked discount in the quantity of myofibres within the older muscle (478,000 vs. 364,000). This same group later verified this proof with a similar forty-three full cross sections of VL, from elderly men aged 15–83 years, and noted a decrease in total muscle length from 20-80 years of age of about 40%, largely associated with a 39% decrease within the range of fibres throughout the same age group. It’s worth noting that muscle fibre loss now did not account for the whole lot of total muscle loss, as smaller (i.e., atrophic) fibres have been also located in older muscle groups, in addition to the truth that there was a ∼20% more amount of noncontractile material inside the older muscle, which could artificially inflate total muscle CSA [68].
The nature of such specific anatomical counts and estimates explains why they are so uncommon; however further studies have made estimates by dividing mean fibre CSA into overall muscle CSA. In a 12-year longitudinal study of nine men, prepared reports that suggested an average 14.7% decrease in quadriceps CSA (from 65 to 77 years) with no decrease in individual fibre CSA, suggesting fibre loss was responsible for the complete muscle atrophy [69]. In relation to muscle loss and force generating potential, Jubrias et al suggested a 21% decline in general muscle size between 65–80 years, with a 39% decrease in force, equating to a 21% decline in specific force (i.e., force normalized to muscle size) [70]. Thus, the age-associated decreases in force generating potential cannot be defined completely by a decrease in muscle size - probably suggesting deleterious neuromuscular remodeling.
In a study estimating VL fibre, the quantity from biopsy and general muscle CSA, data from 31 young (∼22 y) and 40 old (∼72 y) men and women showed the age-associated difference in total muscle size was due, in almost equal quantities, to fibre atrophy and a reduction in the quantity of fibres in the old [71]. But this evidence is not always equivocal, as the same techniques showed the difference in fibre number in bicep brachii (BB) between young (21±2 years) and old (82±2 years) to be minimal (253,000 vs 234,000) [72]. This discrepancy may be explained via the differential response to ageing observed in exclusive muscle tissues [73], and the minimal age-associated loss of CSA in BB. Moreover, [45] showed that up to 100% of age-associated entire muscle atrophy in the VL may be explained by fibre atrophy without the need for fibre loss-primarily based on MRI of thigh muscles and determining fibre area of related muscle biopsies [74], although older fibre CSA suggested here were around 25% larger than formerly reported areas [75]. As such, it remains debatable as to the contributions of atrophy and fibre loss in complete muscle atrophy. We propose it is highly likely taking all together that both of them are key elements at play in sarcopenia, and that existing facts are compromised via both methodological and physiological variations. Further indirect evidence of ageassociated fibre loss comes from the maximal compound muscle action potentials (CMAP) of old and young muscles, wherein motor neurons serving the muscle of interest are electrically stimulated in order to elicit a maximal contraction. The electrical activity of this contraction can be estimated through electromyography (EMG) to provide an estimate of the amount of contractile material contained within the recording limits of the EMG electrode. Although not without its limitations, this approach continuously depicts that older muscle has a smaller CMAP than younger muscles in a variety of muscles, along with tibialis anterior (TA), soleus, BB, and VL [76], with a further study revealing no age-associated difference in TA [77]. The assumption made here is that the relatively small volume of muscle recorded from is steady, then the older muscle contains a relatively reduced amount of contractile material, probably due to fewer and smaller muscle fibres, combined with an extended variety of denervated fibres.
Truly, variations in experimental design, technical challenges of methodologies, variant between man or woman muscle tissues, and environmental impacts among topics are all considerations leading to the apparent discrepancies between studies. However, all over again, it would commonly seem that each muscle fibre atrophy and the loss of fibres are notably likely to be factors in age-related atrophy of entire muscle groups.
Quantification of motor unit number in older humans
Loss of muscle fibres is associated with the age-associated decrease in the number of motor units (MU) [78]. Defined as the last functional unit of the motor system, the human MU consists of a cell body in the ventral horn of the spinal cord, the alpha motor neuron [79] including all the muscle fibres it innervates. Again, postmortem anatomical studies have furnished a wealth of informative facts on the effects of age; with there being a subsequent progressive decrease in the number of cell bodies in spinal cord sections of people aged over 60 years, and those aged over 75 years having 30% fewer serving the lower limbs than younger [80].
Human in vivo studies using EMG strategies have also proven an age-associated decline in MU quantity, in small, and larger muscle tissues [78]. The loss of a MU will leave a muscle fibre denervated and more susceptible to atrophy and ultimate loss. But many fibres will be re-innervated through a close by surviving axon. These axonal sprouts originate from non-myelinated regions of the axon and can ‘rescue’ a denervated fibre in an attempt to preserve muscle mass, termed MU remodeling [75]. Even though the proof strongly indicates an association between age related MU remodeling and fibre loss, the proposed mechanisms are not absolutely in settlement. The problem begins to occur in the cellular body inside the spinal twine, somewhere alongside the axon, or it originates within the myofibre, inflicting denervation and propagating along the alpha motor neuron in a retrograde way. It is uncertain if denervation is a cause or a consequence of fibre loss or not.
Putative mechanisms of neuromuscular remodeling in older humans
Majority of the more precisely detailed mechanistic data has been generated from rodent models and has focused on the neuromuscular junction (NMJ); the synapse between motor neuron and muscle fibre. The relationship of the NMJ and muscle fibre in this regard can be explained in 3 stages. First of all, with whole innervation, myonuclei close to the synapse express genes involved in NMJ maintenance and renovation (MuSK), that are suppressed in non-synaptic myonuclei. Secondly, the initial denervation proteasomal pathways are up regulated in all myonuclei. Thirdly, after prolonged denervation, there may be an inhibition of autophagy and an increase in the rate of protein synthesis (via mTORC1) [81]. Therefore, a denervated fibre will instantly begin to atrophy, but will continue to exist for an undefined amount of time, and these have been observed in a large number of human biopsies [82]. Similarly, associations of age-related denervation and impaired reinnervation have been developed in animal models, inclusive of alterations in oxidative stress [83], dysregulation of sterol metabolism in the nervous system, conversion of voltagegated sodium channels on fibre membranes and a decrease in the number of key maintenance proteins such as PGC1-a [84].
The quantity of SCs gets reduced with age [85], and their involvement extends beyond the maintenance of the fibre; they act as a source of postsynaptic myonuclei and reduction in their quantity results in reduced maintenance of this area and ultimately degeneration of the NMJ [86], and poor or negative fibre regeneration following reinnervation [87]. In addition to this, terminal Schwann cells are implicated within the remodeling of MUs by starting and guiding axonal sprouts and are regarded to develop and expand impairments with increasing age [88]. However, it is not entirely clear in all instances if these associations result from a cause or a consequence of denervation. Moreover, in many animal studies of this nature the measured response to denervation has followed nerve sectioning or ligation, therefore caution must be employed for the reason that entire muscle denervation may promote the onset of various pathological pathways to that following repeated cycles of de/reinnervation of individual fibres. In human studies, lifelong exercise has been recommended to minimise muscle loss [89] and prevent the age-associated lack of MU number, and presumably fibre number in the TA of old (64 years) but not too old (79 years) athletes [90]. However, a further detailed study found masters athletes (69 years) had a similar number of MUs in the TA as age matched controls [78]. Although it is unlikely that exercise and workouts preserve the number of MU, it could enhance the capacity to reinnervate denervated fibres with a view to preserve muscle fibre number, however this mainly comes from biopsy studies which show increased fibre type grouping in master athletes [91]. Although interesting, this grouping is indicative of a shift in fibre type composition (type I/II; an unbalanced ratio of fibre kind composition would boom the probability of observing increased fibre type groupings) and does not directly prove the grouped fibres belong to the similar MU. It is clear that the notion of motor unit plasticity regarding the prevention of muscle fibre loss in human beings is disproportionately underexplored and possible therapeutic targets in ageing (and diseases) and healing agents warrants further investigation.
Metabolic consequences of aging
The series of changes in the physiology of a person over time is correlated with aging. The aging rates differ between organisms and are genetically dependent [92]. While researchers have already shown that calorie reduction improves lifespan, ageing is still by far the greatest major risk factor for cardiovascular attacks, strokes, cancers, diabetes or most chronic diseases [93]. Evans identified ageing’s metabolic changes as a major contributor to sarcopenia [5]. The age-related changes inherent to the skeletal muscle tend to lead to a reduction in muscle mass and strength, however, extrinsic factors are also significant. Aging species usually have lower levels of food consumption, physical activity, and anabolic hormones (including sex hormones, growth hormones and Insulin-like Growth Factor 1 or IGF1) than younger species, as well as neuronal loss (i.e., fibre denervation) and reduced regenerative ability (i.e., satellite stem cell dysfunction). Increase of the susceptibility to a variety of highly catabolic diseases (e.g., cancer, heart failure, spinal cord injury) that are also more common in the elderly population can also be identified. Failure in regulation of whole-body metabolic health, removal of post-prandial glucose and hepatic gluconeogenesis [94] processes can cause disruption in homeostasis, e.g., hyperglycemia or muscle catabolism. Insulin sensitivity, which is shown to be normal in older individuals, is a key component of metabolic syndrome [95].
Basically, metabolic consequences lead to certain disorders. Cachexia is long known as a disorder associated with a variety of chronic diseases and acute medical conditions. Cachexia was described as a complex metabolic syndrome correlated with underlying disease and described by muscle loss even without any loss of fat mass. Sarcopenia is the age-related weakening of skeletal muscle and function with a life-long process of dynamic and multifactorial etiology [5]. Aging is related to higher levels of pro-inflammatory cytokines believed to change the function of insulin. Here, evidence defining age-related changes in obesity and metabolism are important factors in a destructive cycle that can intensify the ageing process and the initiation of age-related diseases [96].
Possible Interventions to Minimize Age Related Muscle Loss
Treating somatopause
Aging is a dynamic process characterized by the aspects of time, society and psychophysiology. While the temporal one is common and instinctively obvious, representing the passage of time, the other two dimensions are intertwined and have some particularities related to gender [97]. It has been proposed that the decline in lean body weight and growth of adipose tissue that occurs with ageing is due in part to the age-related decline in growth hormone (GH) secretion and insulin-like growth factor-1 (IGF-1), also known as somatomedin C, which is developed in response to GH by the liver and other tissues. This drop in the GH-IGF-1 axis’s secretory activity has been called somatopause or ageing hyposomatotropism.
In collaborative efforts with Professor Kjaer ‘s group in Copenhagen, experiments with the author’s group found that strength enhanced with growth hormone administration, which apparently increases the rate of the primary IGF-I transcript, and there was a link between increased MGF levels and muscle mass measured by MRI scanning. A research by showed that the expression of IGF-I splice variants was more affected by mechanical factors than endocrine activity in hypophysectomized rat muscles [98]. This reconfirmed a study conducted by Goldberg [99], who used hypophysectomized rats and demonstrated that they still underwent hypertrophy when muscles are under mechanical pressure, suggesting that muscle mass and strength control are regulated at the cellular muscle level. As IGF-I is carcinogenic at relatively high concentrations, elevated GH levels are associated with increased IGF-I and increased cancer risk, even if it has been reported as suppressing protein breakdown. Acromegalic patients whose pituitary gland is hyperactive are at greater risk of cancer.
It can be seen that ageing-caused (patho) physiological changes within the human body are close to those found in GH deficiency, whereas GH treatment was found effective in terms of optimum consumption of oxygen and overall quality of life [100]. This may be an issue for young athletes and bodybuilders and this type of violence is becoming a problem for the antidoping agencies. Therefore, there are major risks associated with trying to elevate serum GH levels beyond what is normal for an artificial elevation of GH or IGF in a specific age group. The levels of many hormones that are involved in muscle mass maintenance are known to drop significantly with age, including testosterone as well as GH [101].
In addition to hormone supplementation techniques in a somatopausal symptom recovery service, a certain number of pharmaceuticals are produced for this reason. Cerebral selective cholinesterase inhibitors such as rivastigmine and donepezil, have therefore been shown to be helpful in enhancing GH release and IGF-1 levels in elderly humans, but their long-term effectiveness and safety have remained uncertain [102]. In addition, alfacalcidol (1-hydroxycholecalciferol), a vitamin D analogue, has had multi - factorial effects on the familiar somatopausal symptoms [103].These compounds activate various androgen receptors, in particular the testosterone receptor, as the endocrine system is complex and the risks of causing unintended side effects are the concerns why the pharmaceutical industry is trying to improve SARMS and have already made their way into doping in some sports matches [101].
Blocking negative regulation of muscle mass
A TGF- beta family member (Transforming growth factor beta / TGF-β) named myostatin acts as a negative regulatory factor for skeletal muscle mass. As the name suggests, this negative regulation factor is mainly responsible for reducing muscle growth. Certain breeds of cattle like Belgian blue (which show considerable muscle hypertrophy) goes into an allelic series of mutations which in result causes loss of functions of myostatin gene known as “Double muscling”, a few double muscled human subjects are also seen. In myostatin knockout mouse, it is reported that the “extra nuclei’’ is needed in order to increase in size or to repair myofibres of skeletal muscle i.e., postmitotic tissue [104]. Inactivation of postnatal myostatin by gene targeting results in myostatin knockout. Either by deletion of mstn gene (which provides instructions for making myostatin, a protein) [105] or by medicative inhibition of myostatin activity results in picky postnatal loss of myostatin signaling which cause significant muscle fibre hypertrophy, demonstrating, in adult mice, for the regulation of muscle homeostasis, myostatin plays a key role [106]. Partially knocking down/knocking out myostatin is also concluded as not a good strategy for therapy of muscle loss as it hinders respiratory and cardiovascular function as well as it is also associated with loss of oxidative capacity of muscle [107]. Taking a case study (Figure 1) [108].
Figure 1: A study on aging weightlifters reported that when they resume their exercises.

Appealingly, a study on aging weightlifters reported that when they resume their exercises, myostatin level decreases. Plainly if the use of anti-myostatin results in increase of mass without an increase in strength then it would handicap rather than helping elder individuals.
Exercise and decreasing ability to respond to active muscle stretch during aging
In an experiment involving rats and human subjects, a major intrinsic effect is increased load, but this effect becomes dulled during aging. Also, in studies on older human subjects, it was found to be associated with reduced MGF and IGF-1Ea which will be improved by administration of GH which would increase the expression of the IGF-I gene [109]. We understand more about the endocrine aspects than the physical signaling and signaling molecules involved in muscle adaptation involved in maintaining and repairing our muscles as we grow older.
The detection of mechanical strain is believed to involve focal adhesion kinases (FAKs) that are activated by means of mechano transducers systems that connect the extremely long titin molecules which run through the myofibrils to the tendons. These also connect in 3 dimensions as the myofibrillar system is connected to the encompassing basal lamina and response elements, and when these are absent, for example, dystrophin or defective is responsible for the muscle wasting conditions. The connective tissue of the tendons and ligaments additionally transmits the forces generated by the use of sarcomeres of the muscles to tendons, bones, and ligaments. These mechanical forces generated by skeletal musculature are as a consequence also involved in maintaining the complete musculoskeletal system. Evidently as we grow older this mechano transduction system will become less sensitive because of the reduced compliance of the connective tissue because of crosslinking of the collagen which appears to occur in all tissues [110].
The experiments that preceded the discovery of MGF now offer good prospects and accurate potentialities for its use as a therapeutic agent for treating age-associated muscle loss as well as muscle cachexia in a variety of diseases. Despite the fact that there seems to be more interest in its use as a doping agent as it is available over the internet, it is now being produced using recombinant E. coli methods. Consequently, it will become pretty cheaper. Unfortunately, at some stage in getting old the muscle tissue grows to be less compliant and emerges as much less capable of producing MGF [109]. As with different tissues muscle mass end up being much less compliant which apparently occurs because of cross-linking within the connective tissues and probably other factors, and in a long running mouse experiments, it was confirmed that regular exercise advanced muscle compliance while growing older however it was still not as exact as in younger mouse muscle groups [110]. Also, in diseases like muscular dystrophies, the impairment is often the absence or wrong conformation of the certain linking molecules like dystrophin that most probably results due to incapability to produce MGF. From a physiological point of view this is of substantial interest as the initiation of the activation of the IGF-I gene and the switch in splicing to produce MGF ought to contain a mechano transduction system.
The detection of mechanical strain is believed to involve focal adhesion kinases (FAKs) which alter conformation when stretched which phosphorylate other signal molecules [111]. The impairment of mechano signaling seems to be faulty in diseases like the muscular dystrophies wherein splicing of the IGF-I gene is deficient but transfer of normal mesenchymal stem cells into the dystrophic mouse restored its potential to produce MGF [112]. Therefore, there is probably good potential in using therapeutic compounds such as MGF for treating age-associated muscle loss in addition to muscle cachexia in a variety of diseases. Sadly, at the present it appears to be of more interest in its use as a doping agent. Therefore, it can become incredibly less expensive.
Resistance and endurance training
The reduction in muscle strength is subordinate to the development of reduced muscle mass and protein. The reduced number of mitochondria and the corresponding reduction in mitochondrial-based aerobic enzymes could lead to a significant reduction in endurance [113]. Specific neuromuscular and cardiovascular modifications are facilitated by strength and endurance training. Muscle hypertrophy, improved recruitment capacity of the motor unit and motor unit firing rate [114] are the modifications caused by strength training. These neuromuscular changes contribute to enhanced muscle strength and development of control [115]. Endurance training, on the other hand, produces central and peripheral adaptations that enhance cardiovascular functioning and the ability of skeletal muscles through oxidative metabolism to generate energy.
Muscles are entirely capable of adapting to training in resistance. For example, men over 66 years of age who trained for 12 weeks by lifting 80 % of their 1 RM (repetition maximum) encountered strength gains of about 5 percent per day, which is close to what is recorded in many young males. A resistance training programme in a group of individuals where the eligibility criteria were 90 years of age did show that strength as well as the cross-sectional area of the thigh muscles could be enhanced even at this advanced age, both contributing to progress in functional mobility [116]. The clinical significance of the neuromuscular adaptations produced by resistance training is their effect on daily living activities, especially when high-speed activity training is performed in the concentric phase [117].
Increased mitochondrial biogenesis, myoglobin content, capillary density, substrate stocks, oxidative enzyme activities and increased maximum cardiac production are included in endurance training adaptations [118] In fact, endurance training can cause minor increases in muscle strength, particularly when practiced on the ergometer cycle [119]. Although the loss of resistance and endurance is expected to occur across the period of life, keeping older individuals healthy remains significant. With greater exercise, cardiovascular health is significantly enhanced and, in exchange, increased weakness and death are correlated with less physical activity. Through increased enzymatic protein output and capillary density for higher muscle metabolic demand, as well as improved contractile protein production, training will help muscles meet the complex demands of increased activity to allow for higher contracting stress. The age-related impacts on skeletal muscle are generally reversible for many patients [120].
Beyond physical activity
Sarcopenia is a complicated method that includes a lot more than just simply age-associated decreases in physical activity. When a person grows old, the physiology of the system works together to achieve the principal function of the musculoskeletal system. As a consequence, age-related modifications in those systems, mainly people who have an effect on neurological, inflammatory, and hormonal behavior is directly related to Sarcopenia [121].
Nutrition is one of the most difficult components of muscle physiology that includes eating behavior, diet, and digestion [121]. The main anabolic stimuli for muscle protein synthesis are protein consumption and physical activity. The optimal dietary protein should be 1.0-1.2 g/kg (body weight)/day with an optimal repartition over each daily meal or 25-30 g of excessive fine quality protein per meal is recommended to prevent sarcopenia [20]. The role of sex hormones in muscle physiology is a crucial subject matter associated with adaptation with age. Androgen, any of a group of hormones that primarily influence the growth and development of the male reproductive system. The predominant and most active androgen is testosterone, which is produced by the male testes it contributes to the growth in youth which maintains the muscle mass and other tissue and includes bones. Androgen levels decline with age in both male and female [121].
The anabolic actions of testosterone on muscle have been known for over 60 years, and alternative research in younger hypogonadal guys have proven that testosterone can enhance energy and muscular tissues. The supraphysiological substitute of testosterone in younger eugonadal men has additionally been proven to increase muscular tissues and enhance power. Studies of testosterone replacement in healthy older men with relative testosterone deficiency have demonstrated some modest enhancement in muscular tissues and power. In women, lower levels of estrogen lead to increasingly fragile bones and decreased muscle mass; this contributes to decreased activity level and this leads to hormone replacement therapy. Increased rates of prostate cancer, erythrocytosis, adverse cardiovascular events, and a host of other hormonal and emotional adverse events in men, has been associated with testosterone replacement therapy [122]. There are higher rates of cancer and venous thromboembolism when women are treated with estrogen replacement [121].
Conclusion
Term aging describes the frailty in muscle mass or the loss of muscle mass and strength. Also known by the term sarcopenia. In mammals (including humans) and Drosophila, aging in muscle is influenced by muscle regrowth via many responsible extrinsic and intrinsic factors while no growth capacity has been seen in adult Drosophila because of their postmitotic origin, respectively. As loss of muscle mass and muscle functions occur with age, two major influences i.e., muscle fibre atrophy and muscle fibre loss take place. These two influences play a key role in maintaining muscle atrophy and if any dysfunction at the level of the whole muscle. As we age our body goes into several metabolic changes like muscle synthesize less protein and muscle degradation is also seen and hence, we concluded in this review that muscle protein turnover and their repairing ability get affected with age. In this respect, some possible interventions to minimize loss of muscle mass are taken, like by treating somatopause one can maintain their muscle mass balance because GH is markedly dropped during somatopause, and also one can improve their muscle mass production by blocking myostatin that is treated as negative regulation of muscle mass. Only by continuing resistance and endurance type of training one can ameliorate a rapid decline in muscle mass and strength. Aging may cause individuals medical and economic crises, so in that respect, many pharmaceutical industries have taken some serious steps toward increasing muscle mass production and strength in elderly people.
References
- Zierer J, Pallister T, Tsai PC, Krumsiek J, Bell JT, et al. (2016) Exploring the molecular basis of age-related disease comorbidities using a multi-omics graphical model. Sci Rep 6: 37646.
- Mitchell WK, Williams J, Atherton P, Larvin M, Lund J, et al. (2012) Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; A quantitative review. Front physiol 3: 260.
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, et al. (2010) Sarcopenia: European consensus on definition and diagnosis: Report of the European working group on sarcopenia in older people. Age Ageing 39(4): 412-423.
- Marzetti E, Lees AH, Wohlgemuth ES, Leeuwenburgh C (2009) Sarcopenia of aging: Underlying cellular mechanisms and protection by calorie restriction. Biofactors 35(1): 28-35.
- Evans WJ (2010) Skeletal muscle loss: Cachexia, sarcopenia, and inactivity. Am J Clin Nutr 91(4): 1123S-1127S.
- Arango-Lopera VE, Arroyo P, Gutiérrez-Robledo LM, Pérez-Zepeda MU, Cesari M (2013) Mortality as an adverse outcome of sarcopenia. J Nutr Health Aging 17(3): 259-262.
- Bainbridge D, Seow H, Sussman J, Pond G, Martelli-Reid L, et al. (2011) Multidisciplinary health care professionals' perceptions of the use and utility of a symptom assessment system for oncology patients. J Oncol Pract 7(1): 19-23.
- Kirkendall DT, Garrett WE (1998) The effects of aging and training on skeletal muscle. Am J Sports Med 26(4): 598-602.
- Argiles JM, Busquets S, Stemmler B, Lopez-Soriano FJ (2015) Cachexia and sarcopenia: Mechanisms and potential targets for intervention. Curr Opin Pharmacol 22: 100-106.
- Demontis F, Piccirillo R, Goldberg AL, Perrimon N (2013) Mechanisms of skeletal muscle aging: Insights from Drosophila and mammalian models. Dis Models Mech 6(6): 1339-1352.
- Lauretani F, Russo CR, Bandinelli S, Bartali B, Cavazzini C, et al. (2003) Age-associated changes in skeletal muscles and their effect on mobility: An operational diagnosis of sarcopenia. J Appl Physiol 95(5): 1851-1860.
- Rolland Y, Czerwinski S, Van Kan GA, Morley JE, Cesari M, et al. (2008) Sarcopenia: Its assessment, etiology, pathogenesis, consequences and future perspectives. J Nutr Health Aging 12(7): 433-450.
- Murton AJ (2015) Muscle protein turnover in the elderly and its potential contribution to the development of sarcopenia. Proc Nutr Soc 74(4): 387-396.
- Jang HC (2016) Sarcopenia, frailty, and diabetes in older adults. Diabetes Metab 40(3): 182-189.
- Roubenoff R, Castaneda C (2001) Sarcopenia: Understanding the dynamics of aging muscle. JAMA 286(10): 1230-1231.
- Melton LJ III, Khosla S, Crowson CS, O'Connor MK, O'Fallon WM, et al. (2000) Epidemiology of sarcopenia. J Am Geriatr Soc 48(6): 625-630.
- Volpi E, Nazemi R, Fujita S (2004) Muscle tissue changes with aging. Curr Opin Clin Nutr Metab Care 7(4): 405-410.
- Cesari M, Kritchevsky SB, Baumgartner RN, Atkinson HH, Penninx BWHJ, et al. (2005) Sarcopenia, obesity, and inflammation-results from the trial of angiotensin converting enzyme inhibition and novel cardiovascular risk factors study. Am J Clin Nutr 82(2): 428-434.
- Yanai H (2015) Nutrition for sarcopenia. J Clin Med Res 7(12): 926-931.
- Akın S, Mucuk S, Öztürk A, Mazıcıoğlu M, Göçer Ş, et al. (2015) Muscle function-dependent sarcopenia and cut-off values of possible predictors in community-dwelling Turkish elderly: Calf circumference, midarm muscle circumference and walking speed. Eur J Clin Nutr 69: 1087-1090.
- Kenyon C (2005) The plasticity of aging: Insights from long-lived mutants. Cell 120(4): 449-460.
- Piccirillo R, Demontis F, Perrimon N, Goldberg AL (2014) Mechanisms of muscle growth and atrophy in mammals and Drosophila. Dev Dyn 243(2): 201-215.
- Iinattiniemi S, Jokelainen J, Luukinen H (2009) Falls risk among a very old home-dwelling population. Scand J Prim Health Care 27(1): 25-30.
- Clemson L, Mackenzie L, Ballinger C, Close JC, Cumming RG (2008) Environmental interventions to prevent falls in community-dwelling older people: A meta-analysis of randomized trials. J Aging Health 20(8): 954-971.
- Haddad F, Adams GR (2006) Aging-sensitive cellular and molecular mechanisms associated with skeletal muscle hypertrophy. J Appl Physiol 100(4): 1188-1203.
- Nair KS (2005) Aging muscle. Am J Clin Nutr 81(5): 953-963.
- Musarò A, McCullagh K, Paul A, Houghton L, Dobrowolny G, et al. (2001) Localized Igf-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle. Nat Genet 27(2): 195-200.
- O’Connor KG, Tobin JD, Harman SM, Plato CC, Roy TA, et al. (1998) Serum levels of insulin-like growth factor-I are related to age and not to body composition in healthy women and men. J Gerontol A Biol Sci Med Sci 53(3): M176-182.
- Kenyon CJ (2010) The genetics of ageing. Nature 464: 504-512.
- Breen L, Phillips SM (2011) Skeletal muscle protein metabolism in the elderly: Interventions to counteract the ‘anabolic resistance’ of ageing. Nutr Metab 8: 68.
- Tomas FM, Murray AJ, Jones LM (1984) Interactive effects of insulin and corticosterone on myofibrillar protein turnover in rats as determined by N-methylhistidine excretion. Biochem J 220(2): 469-479.
- May RC, Kelly RA, Mitch WE (1986) Metabolic acidosis stimulates protein degradation in rat muscle by a glucocorticoid-dependent mechanism. J Clin Investig 77(2): 614-621.
- Fujisawa K (1975) Some observations on the skeletal musculature of aged rats: Part 2. Fine morphology of diseased muscle fibres. J Neurol Sci 24(4): 447-469.
- Marzetti E, Leeuwenburgh C (2006) Skeletal muscle apoptosis, sarcopenia and frailty at old age. Exp Gerontol 41(12): 1234-1238.
- Garcia AM, Calder RB, Dollé ME, Lundell M, Kapahi P, et al. (2010) Age-and temperature-dependent somatic mutation accumulation in Drosophila melanogaster. PLoS Genet 6(5): e1000950.
- Wohlgemuth SE, Seo AY, Marzetti E, Lees HA, Leeuwenburgh C (2010) Skeletal muscle autophagy and apoptosis during aging: Effects of calorie restriction and life-long exercise. Exp gerontol 45(2): 138-148.
- Webster GC, Beachell VT, Webster SL (1980) Differential decrease in protein synthesis by microsomes from aging Drosophila melanogaster. Exp Gerontol 15(5): 495-497.
- Prochniewicz E, Thompson LV, Thomas DD (2007) Age-related decline in actomyosin structure and function. Exp Gerontol 42(10): 931-938.
- Lee JH, Budanov AV, Park EJ, Birse R, Kim TE, et al. (2010) Sestrin as a feedback inhibitor of TOR that prevents age-related pathologies. Science 327(5970): 1223-1228.
- Höök P, Sriramoju V, Larsson L (2001) Effects of aging on actin sliding speed on myosin from single skeletal muscle cells of mice, rats, and humans. Cell Physiol 280(4): C782-788.
- Miller MS, Lekkas P, Braddock JM, Farman GP, Ballif BA, et al. (2008) Aging enhances indirect flight muscle fiber performance yet decreases flight ability in Drosophila. Biophys J 95(5): 2391-2401.
- Webb S, Tribe MA (1974) Are there major degenerative changes in the flight muscle of ageing diptera? Exp gerontol 9(1): 43-49.
- Braun T, Gautel M (2011) Transcriptional mechanisms regulating skeletal muscle differentiation, growth and homeostasis. Nat Rev Mol Cell Biol 12(6): 349-361.
- Wilkinson DJ, Piasecki M, Atherton PJ (2018) The age-related loss of skeletal muscle mass and function: Measurement and physiology of muscle fibre atrophy and muscle fibre loss in humans. Ageing Res Rev 47: 123-132.
- Atherton PJ, Etheridge T, Watt PW, Wilkinson D, Selby A, et al. (2010) Muscle full effect after oral protein: Time-dependent concordance and discordance between human muscle protein synthesis and mTORC1 signaling. Am J Clin Nutr 92(5): 1080-1088.
- Atherton PJ, Smith K (2012) Muscle protein synthesis in response to nutrition and exercise. J Physiol Paris 590(5): 1049-1057.
- Wall BT, Snijders T, Senden JM, Ottenbros CLP, Gijsen AP, et al. (2013) Disuse impairs the muscle protein synthetic response to protein ingestion in healthy men. J Clin Endocrinol Metab 98(12): 4872-4881.
- Wilkes EA, Selby AL, Atherton PJ, Patel R, Rankin D, et al. (2009) Blunting of insulin inhibition of proteolysis in legs of older subjects may contribute to age-related sarcopenia. Am J Clin Nutr 90(5): 1343-1350.
- Abdulla H, Smith K, Atherton PJ, Idris I (2016) Role of insulin in the regulation of human skeletal muscle protein synthesis and breakdown: A systematic review and meta-analysis. Diabetologia 59(1): 44-55.
- Rudrappa SS, Wilkinson DJ, Greenhaff PL, Smith K, Idris I, et al. (2016) Human skeletal muscle disuse atrophy: effects on muscle protein synthesis, breakdown, and insulin resistance - A qualitative review. Front physiol 7: 361.
- Atherton PJ, Wilkinson DJ, Smith K (2016) Chapter 9 - feeding modulation of amino acid utilization: Role of insulin and amino acids in skeletal muscle. The Molecular Nutrition of Amino Acids and Proteins pp. 109-124.
- Wilkinson DJ, Brook MS, Smith K, Atherton PJ (2017) Stable isotope tracers and exercise physiology: past, present and future. J Physiol Paris 595(9): 2873-2882.
- Darmaun D (1994) Méthodes d'études du métabolisme des acides aminés in vivo chez l'homme: Approches cinétiques par les isotopes stables. Nutr Clin Metab 8(2): 81-91.
- Liu Z, Barrett EJ (2002) Human protein metabolism: Its measurement and regulation. American Journal of Physiology-Endocrinology and Metabolism 283(6): E1105-E1112.
- Poortmans JR, Carpentier A, Pereira-Lancha LO, Lancha Jr A (2012) Protein turnover, amino acid requirements and recommendations for athletes and active populations. Braz J Med Biol Res 45(10): 875-890.
- Vingren JL, Kraemer WJ, Ratamess NA, Anderson JM, Volek JS, et al. (2010) Testosterone physiology in resistance exercise and training: the up-stream regulatory elements. Sports Med 40(12): 1037-1053.
- Phillips SM (2012) Strength and hypertrophy with resistance training: Chasing a hormonal ghost. Eur J Appl Physiol 112(5): 1981-1983.
- Brasse-Lagnel C, Lavoinne A, Husson A (2009) Control of mammalian gene expression by amino acids, especially glutamine. FEBS J 276(7): 1826-1844.
- Inoki K, Li Y, Xu T, Guan KL (2003) Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev 17(5): 1829-1834.
- Wackerhage H, Ratkevicius A (2008) Signal transduction pathways that regulate muscle growth. Essays Biochem 44: 99-108.
- Long X, Lin Y, Ortiz-Vega S, Yonezawa K, Avruch J (2005) Rheb binds and regulates the mTOR kinase. Curr Biol 15(8): 702-713.
- Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, et al. (2010) Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell 141(2): 290-303.
- Marimuthu K, Murton AJ, Greenhaff PL (2011) Mechanisms regulating muscle mass during disuse atrophy and rehabilitation in humans. J Appl Physiol 110(2): 555-560.
- Kelleher AR, Kimball SR, Dennis MD, Schilder RJ, JeffersonLS (2013) The mTORC1 signaling repressors REDD1/2 are rapidly induced and activation of p70S6K1 by leucine is defective in skeletal muscle of an immobilized rat hindlimb. 304(2): E229-E236.
- Keller P, Vollaard NB, Gustafsson NB, Gallagher IJ, Sundberg CJ, et al. (2011) A transcriptional map of the impact of endurance exercise training on skeletal muscle phenotype. J Appl Physiol 110(1): 46-59.
- Nielsen S, Scheele C, Yfanti C, Akerström T, Nielsen AR, et al. (2010) Muscle specific microRNAs are regulated by endurance exercise in human skeletal muscle. J Physiol Paris 588(Pt 20): 4029-4037.
- Lexell J, Henriksson-Larsén K, Winblad B, Sjöström M (1983) Distribution of different fiber types in human skeletal muscles: Effects of aging studied in whole muscle cross sections. Muscle Nerve 6: 588-595.
- Frontera WR, Hughes VA, Fielding RA, Fiatarone MA, Evans WJ, et al. (2000) Aging of skeletal muscle: A 12-yr longitudinal study. J Appl Physiol 88(4): 1321-1326.
- Jubrias SA, Odderson IR, Esselman PC, Conley KE (1997) Decline in isokinetic force with age: Muscle cross-sectional area and specific force. Pflugers Archiv 434(3): 246-253.
- McPhee JS, Cameron J, Maden-Wilkinson T, Piasecki M, Yap MH, et al. (2018) The contributions of fibre atrophy, fibre loss, in situ specific force and voluntary activation to weakness in sarcopenia. J Gerontol a Biol Sci Med Sci 73(10): 1287-1294.
- Klein CS, Marsh GD, Petrella RJ, Rice CL (2003) Muscle fiber number in the biceps brachii muscle of young and old men Muscle Nerve. Muscle Nerve 28(1): 62-68.
- Piasecki M, Ireland A, Piasecki J, Stashuk DW, Swiecicka A, et al. (2018) Failure to expand the motor unit size to compensate for declining motor unit numbers distinguishes sarcopenic from non-sarcopenic older men. J Physiol Paris 596(9): 1627-1637.
- Nilwik R, Snijders T, Leenders M, Groen BBL, van Kranenburg J, et al. (2013) The decline in skeletal muscle mass with aging is mainly attributed to a reduction in type II muscle fiber size. Exp gerontol 48(5): 492-498.
- Gouzi F, Maury J, Molinari N, Pomiès P, Mercier J, et al. (2013) Reference values for vastus lateralis fiber size and type in healthy subjects over 40 years old: A systematic review and metaanalysis. J Appl Physiol 115(3): 346-354.
- Piasecki M, Ireland A, Stashuk D, Hamilton-Wright A, Jones DA, et al. (2016) Age-related neuromuscular changes affecting human vastus lateralis. J Physiol Paris 594(16): 4525-4536.
- Hourigan ML, McKinnon NB, Johnson M, Rice CL, Stashuk DW, et al. (2015) Increased motor unit potential shape variability across consecutive motor unit discharges in the tibialis anterior and vastus medialis muscles of healthy older subjects. Clin Neurophysiol 126(12): 2381-2389.
- Piasecki M, Ireland A, Jones DA, McPhee JS (2016) Age-dependent motor unit remodeling in human limb muscles. Biogerontology 17(3): 485-496.
- Hof PR, Trapp BD, De Vellis J, Claudio L, Colman DR (2004) Chapter 1-Cellular Components of Nervous Tissue.
- Mittal KR, Logmani FH (1987) Age-related reduction in 8th cervical ventral nerve root myelinated fiber diameters and numbers in man. J Gerontol 42(1): 8-10.
- Tintignac LA, Brenner HR, Rüegg MA (2015) Mechanisms regulating neuromuscular junction development and function and causes of muscle wasting. Physiol Rev 95(3): 809-852.
- Spendiff S, Vuda M, Gouspillou G, Aare S, Perez A, et al. (2016) Denervation drives mitochondrial dysfunction in skeletal muscle of octogenarians. J Physiol Paris 594(24): 7361-7379.
- Vasilaki A, Richardson A, Van Remmen H, Brooks SV, Larkin L, et al. (2017) Role of nerve-muscle interactions and reactive oxygen species in regulation of muscle proteostasis with ageing. J Physiol Paris 595(20): 6409-6415.
- Gouspillou G, Picard M, Godin R, Burelle Y, Hepple RT (2013) Role of peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) in denervation-induced atrophy in aged muscle: facts and hypotheses. Longev Healthspan 2(1): 13.
- Verdijk LB, Snijders T, Drost M, Delhaas T, Kadi F, et al. (2014) Satellite cells in human skeletal muscle; from birth to old age. Age 36(2): 545-557.
- Liu W, Klose A, Forman S, Paris ND, Wei-LaPierre L, et al. (2017) Loss of adult skeletal muscle stem cells drives age-related neuromuscular junction degeneration. Elife 6: e26464.
- Dedkov EI, Kostrominova TY, Borisov AB, Carlson BM (2001) Reparative myogenesis in long-term denervated skeletal muscles of adult rats results in a reduction of the satellite cell population. Anat Rec 263(2): 139-154.
- Saheb-Al-Zamani M, Yan Y, Farber SJ, Hunter DA, Newton P, et al. (2013) Limited regeneration in long acellular nerve allografts is associated with increased Schwann cell senescence. Exp Neurol 247: 165-177.
- Mckendry J, Breen L, Shad BJ, Greig CA (2018) Muscle morphology and performance in master athletes: A systematic review and meta-analyses. Ageing Res Rev 45: 62-82.
- Power GA, Allen MD, Gilmore KJ, Stashuk DW, Doherty TJ, et al. (2016) Motor unit number and transmission stability in octogenarian world class athletes: Can age-related deficits be outrun? J Appl Physiol 121(4): 1013-1020.
- Zampieri S, Pietrangelo L, Loefler S, Fruhmann H, Vogelauer M, et al. (2015) Lifelong physical exercise delays age-associated skeletal muscle decline. J Gerontol a Biol Sci Med Sci 70(2): 163-173.
- Bowen RL, Atwood CS (2004) Living and dying for sex. A theory of aging based on the modulation of cell cycle signaling by reproductive hormones. Gerontol 50(5): 265-290.
- Slawik M, Vidal-Puig AJ (2006) Lipotoxicity, overnutrition and energy metabolism in aging. Ageing Res Rev 5(2): 144-164.
- Brook MS, Wilkinson DJ, Mitchell WK, Lund JN, Phillips BE, et al. (2016) Synchronous deficits in cumulative muscle protein synthesis and ribosomal biogenesis underlie age-related anabolic resistance to exercise in humans. J Physiol Paris 594(24): 7399-7417.
- Arum O, Boparai RK, Saleh JK, Wang F, Dirks AL, et al. (2014) Specific suppression of insulin sensitivity in growth hormone receptor gene‐disrupted (GHR‐KO) mice attenuates phenotypic features of slow aging. Aging cell 13(6): 981-1000.
- Jura M, Kozak LP (2016) Obesity and related consequences to ageing. Age 38(1): 23.
- Ajdžanovic VZ, Trifunovic S, Miljic D, Šošic-Jurjevic B, Filipovic B, et al. (2018) Somatopause, weaknesses of the therapeutic approaches and the cautious optimism based on experimental ageing studies with soy isoflavones. EXCLI J 17: 279-301.
- Yamaguchi A, Fujikawa T, Shimada S, Kanbayashi I, Tateoka M, et al. (2006) Muscle IGF-I Ea, MGF, and myostatin mRNA expressions after compensatory overload in hypophysectomized rats. Pflügers Archiv 453(2): 203-210.
- Goldberg AL (1967) Work-induced growth of skeletal muscle in normal and hypophysectomized rats. Am J Physiol 213(5): 1193-1198.
- Clayton P, Gleeson H, Monson J, Popovic V, Shalet SM, et al. (2007) Growth hormone replacement throughout life: insights into age-related responses to treatment. Growth Horm IGF Res 17(5): 369-382.
- Nigam Y, Knight J, Bhattacharya S, Bayer A (2012) Physiological changes associated with aging and immobility. J Aging Res.
- Obermayr RP, Mayerhofer L, Knechtelsdorfer M, Mersich N, Huber ER, et al. (2005) The age-related down-regulation of the growth hormone/insulin-like growth factor-1 axis in the elderly male is reversed considerably by donepezil, a drug for Alzheimer's disease. Exp Gerontol 40(3): 157-163.
- Schacht E, Richy F, Reginster J (2005) The therapeutic effects of alfacalcidol on bone strength, muscle metabolism and prevention of falls and fractures. J Musculoskeletal Neuronal Interact 5(3):273-284.
- McMahon CD, Popovic L, Oldham JM, Jeanplong F, Smith HK, et al. (2003) Myostatin-deficient mice lose more skeletal muscle mass than wild-type controls during hindlimb suspension. Am J Physiol Endocrinol Metab 285(1): E82-E87.
- Grobet L, Pirottin D, Farnir F, Poncelet D, Royo LJ, et al. (2003) Modulating skeletal muscle mass by postnatal, muscle‐specific inactivation of the myostatin gene. Gen 35(4): 227-238.
- Wolfman NM, McPherron AC, Pappano WN, Davies MV, Song K, et al. (2003) Activation of latent myostatin by the BMP-1/tolloid family of metalloproteinases. PNAS 100(26): 15842-15846.
- Arthur PF (1995) Double muscling in cattle: A review. Aust J Agric Res 46(8): 1493-1515.
- Goldspink G (2012) Age-related loss of muscle mass and strength. J Aging Res.
- Goldspink G, Yang SY (2004) The splicing of the IGF-I gene to yield different muscle growth factors. Adv Genet 52: 23-49.
- Alnaqeeb MA, Al Zaid NS, Goldspink G (1984) Connective tissue changes and physical properties of developing and ageing skeletal muscle. J Anat 139(Pt 4): 677-689.
- Klossner S, Durieux AC, Freyssenet D, Flueck M (2009) Mechano-transduction to muscle protein synthesis is modulated by FAK. Eur J Appl Physiol 106(3): 389-398.
- Moss FP, Leblond CP (1971) Satellite cells as the source of nuclei in muscles of growing rats. Anat Rec 170(4): 421-435.
- Essen‐Gustavsson B, Borges O (1986) Histochemical and metabolic characteristics of human skeletal muscle in relation to age. Acta Physiol Scand 126(1): 107-114.
- Kamen G, Knight CA (2004) Training-related adaptations in motor unit discharge rate in young and older adults. J Gerontol A Biol Sci Med Sci 59(12): 1334-1338.
- Peterson MD, Rhea MR, Sen A, Gordon PM (2010) Resistance exercise for muscular strength in older adults: a meta-analysis. Ageing Res Rev 9(3): 226-237.
- Frontera WR, Meredith CN, O'Reilly KP, Knuttgen HG, Evans WJ (1988) Strength conditioning in older men: Skeletal muscle hypertrophy and improved function. J Appl Physiol 64(3): 1038-1044.
- Pereira A, Izquierdo M, Silva AJ, Costa AM, Bastos E, et al. (2012) Effects of high-speed power training on functional capacity and muscle performance in older women. Exp Gerontol 47(3): 250-255.
- Beere PA, Russell SD, Morey MC, Kitzman DW, Higginbotham MB (1999) Aerobic exercise training can reverse age-related peripheral circulatory changes in healthy older men. Circulation 100(10): 1085-1094.
- Cadore EL, Pinto RS, Lhullier FLR, Correa CS, Alberton CL, et al. (2010) Physiological effects of concurrent training in elderly men. Int J Sports Med 31(10): 689-697.
- Faulkner JA, Larkin LM, Claflin DR, Brooks SV (2007) Age‐related changes in the structure and function of skeletal muscles. Clin Exp Pharmacol Physiol 34(11): 1091-1096.
- Siparsky PN, Kirkendall DT, Garrett Jr WE (2014) Muscle changes in aging: Understanding sarcopenia. Sports Health 6(1): 36-34.
- Horstman AM, Dillon EM, Urban RJ, Sheffield-Moore M (2012) The role of androgens and estrogens on healthy aging and longevity. J Gerontol A Biol Sci Med Sci 67(11): 1140-1152.
© 2021 Upadhyay AK. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






