- Submissions

Full Text
Novel Research in Sciences
Covid-19: In Pursuit of a Cure
Jagessar RC1*
Department of Chemistry, South America
*Corresponding author: Jagessar RC, Department of Chemistry, South America
Submission: November18, 2020;Published: January 25, 2021
.jpg)
Volume5 Issue4January, 2021
Abstract
Our world is in a chaotic state at the moment. This stem mostly from the current infectious, highly contagious viral disease, COVID-19, and not to mention global warming and its catastrophic effects. COVID-19 is caused by severe acute respiratory syndrome coronavirus 2, SARS-COV-2, a possible mutant of SARS-COV-1. It was first identified in Wuhan, China in December,2019, though there is speculation that it may have originated elsewhere. It has resulted in a current ongoing pandemic. A person infected with the virus can be symptomatic or asymptomatic. Common symptoms include fever, cough, fatigue, shortness of breath, loss of smell and taste, multi organ failure, septic shock. blood clots etc. The virus is spread primarily between people, during close contact via small droplets, produced by coughing, sneezing and talking, touching one own face after contact with a contaminated surface. It’s also postulated that its spread may also be airborne, requiring particulate matter in the atmosphere for further transmission. Whilst preventative measures for COVID-19, have been implemented by WHO and CDC, there is no known vaccine or specific antiviral treatment to date, even though the first is expected in the late, 2020. Considering that viruses are very much smaller than bacteria and they mutate, it would be best to use and explore drugs combination or herbal mixtures to control and eradicate this disease. A virus is not a bacterium, and so would require herbs that show strong and selective antiviral activity. Over one hundred Pharmaceutical research institutions are currently working to find a cure for this disease.It would require a concerted effort from synthetic organic chemists, natural product chemists, pharmaceutical chemists, medical doctors and other health professionals to find a cure for this planet threatening disease. The best way forward is to explore herbal medications,with a strong and selective anti-herbal activities and to use the current plethora of existing antiviral medications, singly or in combination as first choice, considering the urgent need and then later to resort to the synthesis of novel anti-viral drugs. Some of the antiviral medications at hand are.
Keywords: COVID-19; SARS-COV-2; Wuhan; Symptomatic; Asymptomatic; Pandemic; Mutate; Eradicate
Introduction and Background
COVID-19 is an infectious new disease induced by severe acute respiratory syndrome coronavirus 2 (SARS-COV-2), a novel virus [1] and a possible mutant of SARs-COV-1. The outbreak was first identified in Wuhan, China, in December 2019, though there is speculation that the virus could have originated elsewhere [2,3].The outbreak was declared a Public Health Emergency of International Concern on 30th January 2020 and a pandemic on 11th March by the World Health Organization. As of 15 November 2020, more than 54.1 million cases have been confirmed, with more than 1.31 million deaths [4,5].
The virus is primarily spread between people during close contact via small droplets produced by coughing, sneezing, talking and singing. The droplets usually fall to the ground or onto surfaces, rather than travelling through air over long distances. As of June 2020, research has shown that speech-generated droplets may remain airborne for tens of minutes, providing the opportunity for the virus to be further spread by particulate matter in the atmosphere. People may become infected by touching a contaminated surface and then touching their face [6,7]. COVID-19 has affected humanity significantly. It has resulted in a decline in world’s economy, affected world’s education. Universities, primary and secondary schools have been closed and forced to dispense their mission online. Whilst this has worked well for Universities across the globe, it has not been so in primary and secondary schools. Primary and secondary schools’ curriculum have been modified. COVID-19 has affected the social fabrics of societies and initiated social unrest in some countries. There is thus an urgent need to find a cure for this life-threatening global disease. This paper attempts to give great insight to the direction leading to a cure.
This current pandemic has been the impetus for over one hundred and fifty pharmaceutical companies around the globe to search for the appropriate medications and vaccine. It’s expected that the first vaccine will be out before December 2020. An excellent vaccine is one that shows 100% eradication of the disease and produce little or no side effects and is also one that is sustainable over a long period of time. Pfizer, in early November 2020 has announced, a vaccine to cure COVID-19. However, double vaccination is necessary. Thus, the world awaits the use of Pfizer’s vaccine.
Structure of Virus
Knowing the structure of the virus, is the first step to find a cure for COVID-19, as this would give an idea of the design and structure of the drug molecule. There are more than 6,000 virus species described in detail of the millions of types of viruses in the environment. Viruses are extremely small, approximately 20 - 400 nanometers in diameter and occur in varying shapes and sizes. Similar to bacteria, some viruses have spherical or rod shapes. Other viruses are icosahedral (polyhedron with 20 faces) or helical shaped. Viral shape is determined by the protein coat that encases and protects the viral genome.
A virus particle, also known as a virion, is essentially nucleic acid (DNA or RNA), long strands, enclosed within a protein shell or coat, called the capsid. In addition, there is a lipid outercoat. A capsid is composed of protein subunits called capsomeres. Capsids can have several shapes: polyhedral, rod or complex. Capsids function to protect the viral genetic material from damage. In addition to the protein coat, some viruses have specialized structures. For example, the flu virus has a membrane-like envelope around its capsid.
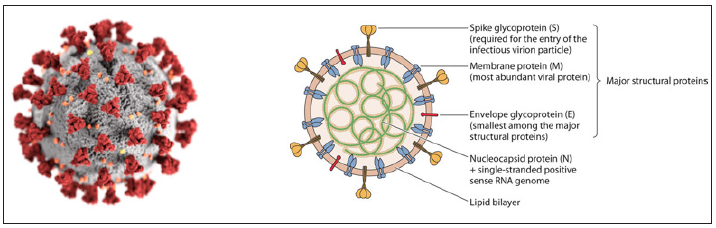
Viruses may have double-stranded DNA, double-stranded RNA, single-stranded DNA or single-stranded RNA. The type of genetic material found in a particular virus depends on the nature and function of the specific virus. The genetic material is not typically exposed but covered by a protein coat known as a capsid. The viral genome can consist of a very small number of genes or up to hundreds of genes depending on the type of virus. Note that the genome is typically organized as a long molecule that is usually straight or circular. All features of the novel SARS‑COV‑2 virus occur in related coronaviruses in nature. SARS‑COV‑2 is closely related to SARS‑COV and is thought to have a zoonotic origin. SARS‑COV‑2 is 96 % identical at the whole genome status of other bat coronavirus samples and 92 % identical to pangolin coronavirus. (Figure 1a) shows the overall structure of a virion, whereas (Figure 1b) shows a detailed structure of SARS-COV-2-virus.
Figure 1: (a) Generalised structure of SARS-COV-2 virus (b) Detailed structure of SARS-CoV-2 virus.
Virus Replication
Viruses can infect all types of life forms, from animals and plants to microorganisms, including bacteria and archaea. Viruses cannot exist independently of other organisms, as they must take over a living cell or host cell in order to reproduce or replicate. When infected, a host cell is forced to rapidly produce thousands of identical copies of the original virus. The virus injects its genetic material into the cell and uses the cell’s organelles to replicate. Once a sufficient number of viruses have been replicated, the newly formed viruses lyse or break open the host cell and move on to infect other cells. This type of viral replication is known as the lytic cycle [8-10].
In Pursuit of a Cure
To date, there are few drugs or vaccine developed to eradicate viruses of which SARS-COV-2 is of no exception. No vaccine has been developed to date. However, over a one hundred and fifty pharmaceutical corporations are pursuing drugs and vaccines to eradicate the SARS COVID-2-virus. However, to eradicate viruses, one has to know the life cycle of the virus, especially the critical steps. Viral replication involves several steps. Antiviral agents, both synthetic and of plant extracts origin can target any of these steps. Antiviral agents are expected to work via the inhibition of viral DNA or RNA synthesis or an inhibition of the activity of viral replication in the host environment or viral genome synthesis. Antiviral agents can get incorporated into viral DNA and causes DNA chain termination. Antiviral agents can be synthetic drugs, vaccines and plant extracts. Antiviral drugs can exert their actions at several stages of viral replication, including adsorption and penetration, nucleic acid synthesis, late protein synthesis, processing and in the final stages of viral packaging and virion release [11].
Amongst the synthetic antiviral drugs currently in use are: Famciclovir, Penciclovir, Docosanol, Trifluride, Ganciclovir, Valganciclovir, Foscarnet, Cidofovir, Abacavir, Didanosine, Emtricitabine, Lamivudine, Stavudine, Tenofovir disoproxil fumarate, Zidovudine, Delavirdine, Efavirenz, Etravirine, Nevirapine, Rilpivirine, Atazavir, Darunavir, Fosamprenavir, Indinavir, Lopinavir, Nelfinavir, Ritonavir, Saquinavir, Tipranavir, Enfuvirtide, Maraviroc etc. Figure 2 shows the structure of some antiviral drugs which can be used singly or in combination with others to fight COVID-19 [8-11].
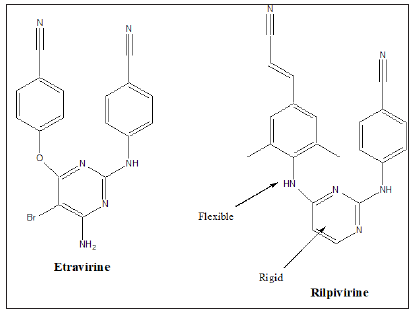
The antiviral drug or drug combinations, selected to eradicate SARS-COV-2 and COVID-19, must encapsulate the virus, analogous to a “lock and key”, (Figure 2). The receptor, drug must show complementary with the virus structure and converge its binding sites on the virus. Since the out coat of viruses are proteinaceous in nature, one would expect favorable-NHCO-hydrogen bonding interactions, that would disrupt the structure of the virus, exudation of its content and death. Since virus mutate, their conformation can change and thus even though a designed drug molecule works well, over a period of time, it may not be useful after a period of time. To compensate for this, many antiviral drug molecules synthesized to date, have a rigid basal synthetic skeleton, to which is attached a flexible arm. So, as the conformation of the virus changes, so too will the drug molecule, allowing for sustainable drug virus interactions and an inactivation of the virus. (Figures 3-5) shows the structure of some antiviral drugs to date, illustrating the rigid base and the flexible arms, that wraps around the virus.
Figure 2: Drug/virus interactions, complementary.
Figure 3: shows the structure of some antiviral drugs, incorporating a rid skeleton and a flexible arm.
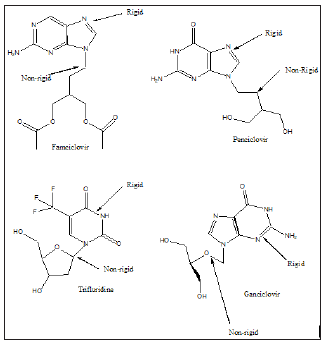
Figure 4: Structure of some antiviral drugs, incorporating a rigid skeleton and a flexible arm.
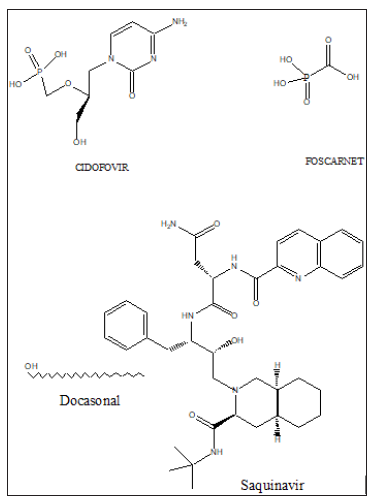
Figure 5: Structure of some antiviral drugs incorporating a rigid base and flexible arm.
Research on COVID-19 Treatments and Medications in Pursuit
Hospital and research labs all over the world are testing many different therapies on corona virus positive patients, with a view to find a potential COVID-medication and vaccine. Some drugs under testing are: Antiprotozoal agent, hydroxychloroquine and chloroquine, antivirals such as Remdesivir and Favipiravir (Avigan), anti-retroviral such as Umifenovir (umi-fen-o-vir), dexamethasone, Tocilizumab (Actemra), Azithromycin, Kaletra (lopinavir/ ritonavir), Tamiflu (Oseltamivir), Colcrys (colchicine), Ivermectin etc. Hydroxychloroquine (HCQ), (Plaquenil) is a medication used to prevent and treat malaria in areas where malaria remains sensitive to chloroquine. Other uses include treatment of rheumatoid arthritis, lupus, and porphyria cutanea tarda. It is taken by mouth. HCQ is being studied to prevent and treat coronavirus disease 2019 (COVID‑19). It prevents endocytosis and has the ability to alkalinizes vacuolar and lysosomal pH, thereby preventing entry of the viral particle into the cell.
Hydroxychloroquine may bind to sialic acid and ACE-2 receptors on the outer cell wall. It also inhibits RNA-polymerase activity and act as a zinc ionophore. Hydroxychloroquine may act as an ion transporter for zinc ion to enter the cell, which then binds to RNA dependent RNA polymerase, enzyme responsible for the replication of RNA [11]. Severe side effects may include allergic reactions, vomiting, headache vision problems, and heart problems. However, the FDA have removed the Emergency Use Authorization (EUA) for hydroxychloroquine and chloroquine for the treatment of COVID-19 (Figures 6-9). Based on some latest research, the FDA determined that these drugs are not likely to be an effective treatment for COVID-19 and that the risks of using them for this purpose might outweigh any benefits. Thus, HCQ is now removed as a drug for use against COVID-19 [12-15].
Figure 6: Etravirine, SARS-COV-2-Complex.
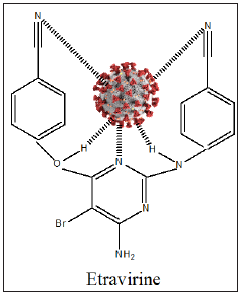
Figure 7: Hydrochloroquinine and Remdesivir.
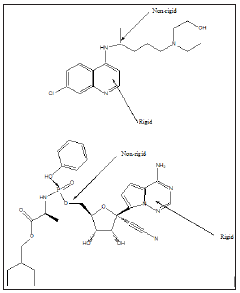
Figure 8:
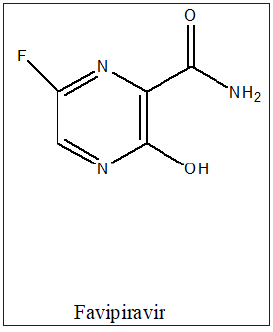
Figure 9:
Remdesivir, (Veklury), is a broad-spectrum antiviral medication developed by the biopharmaceutical company, Gilead Sciences. As of 2020, Remdesivir is being tested as a specific treatment for COVID-19, and has been authorized for emergency use in the US, India, Singapore, and approved for use in Japan and the European Union for people with severe symptoms. It also received approval in the UK in May 2020.
It is a prodrug that is a ribonucleotide analogue inhibitor of viral RNA polymerase. Earlier studies found antiviral activity against several RNA viruses including SARS coronavirus and MERS coronavirus, but it is not approved for any indication. Remdesivir was originally developed to treat hepatitis C and was then tested against Ebola virus disease and Marburg virus disease but was ineffective for all of these viral infections. The most common side effects in people with COVID-19 is nausea, liver inflammation and an infusion-related reaction with nausea, low blood pressure, and sweating [16-18].
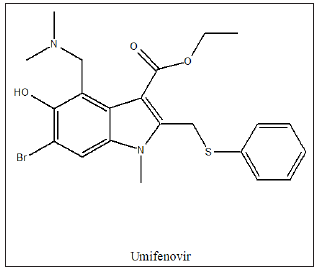
Favipiravir, also known as Avigan, is an antiviral medication used to treat influenza in Japan and China. It is also being studied to treat a number of other viral infections, such as the antiviral drugs (T-1105 and T-1106). It is a pyrazine carboxamide derivative. In vitro studies, have shown that high doses of favipiravir, were able to prevent human cells from being infected with SARS-COV-2 virus. Umifenovir, (Arbidol) is an antiviral treatment for influenza infection used in Russia and China. Although some Russian studies have shown it to be effective. It is not approved by the US FDA for the treatment or prevention of influenza. The drug is claimed to inhibit viral entry into target cells and stimulate the immune response. Interest in the drug has been renewed as a result of the COVID-19 pandemic. It was not as good as favipiravir in helping patients recover in a study from China. It seems to work better than Kaletra at helping patients with COVID-19 eradicate the virus. As of 2020, umifenovir and darunavir were being studied as a potential combination treatment for COVID-19 in China [19,20].
Umifenovir works via inhibition of membrane fusion of influenza virus and prevents contact between the virus and target host cells. Fusion between the viral envelope, surrounding the viral capsid, and the cell membrane of the target cell is inhibited. This prevents viral entry to the target cell, and therefore protects it from infection. Some evidence suggests that the drug’s actions are more effective at preventing infections from RNA viruses than infections from DNA viruses. As well as specific antiviral action against both influenza A and influenza B viruses, umifenovir exhibits modulatory effects on the immune system. The drug stimulates a humoral immune response, induces interferon-production, and stimulates the phagocytic function of macrophages.
Azithromycin (also known as a Z-pak) is an antibiotic used to treat bacterial infections such as bronchitis and pneumonia. It has been shown to have some in vitro activity against viruses like influenza A and Zika but didn’t work against the coronavirus that causes MERS. In one research, azithromycin has been used in combination with hydroxychloroquine for COVID-19. It was reported that 93% of patients cleared the virus after eight days. However, there was no control group, so it cannot be concluded firmly, whether the people cleared the virus on their own or via the medications.
Azithromycin is being studied together with other medications in COVID-19. There is no strong evidence to support combining azithromycin with hydroxychloroquine to treat COVID-19, though such use is being studied. Azithromycin was recently identified as a broad-spectrum therapeutic [21,22]. Tamiflu (Oseltamivir), is an antiviral medication used for influenza flu. Results from a hospital in Wuhan, China were not promising. Nevertheless, several clinical trials are currently looking at Tamiflu in combination with other medications for coronavirus [23].
Colchine (Colcrys) is a medication used for gout. It works in many different ways, including activating anti-inflammatory processes and interfering with cells involved in inflammation. Its speculated that colchicine could work the similarly to tocilizumab (Actemra) in COVID-19 patients, via the prevention of cytokine storm. A clinical trial of 6000 people with COVID-19 infection, funded by the Government of Quebec, began in March 2020 to test the potential efficacy of using colchicine over a 30-day period to reduce disease symptoms [24].
Ivermectin is an oral medication used to treat infections caused by parasites, such as lice and rosacea. Recently, it has been shown via invitro study, that ivermectin can stop SARS-COV-2 virus from replicating. Further studies are necessary to see whether the doses will be safe and effective against the virus in humans. Ivermectin has antiviral effects against several positive single-strand RNA viruses, including SARS-CoV-2. Ivermectin inhibits replication of SARS-CoV-2 in monkey kidney cell culture with an IC50 of 2.2-2.8 μM, making it a possible candidate for COVID-19 drug research.[81] [82] The doses used in cell culture would require 104 larger doses in humans based on this data, which does not look promising as an effective treatment for COVID-19, since such a large dose may be dangerous to humans [25,26].
Galidesivir is a new drug that is currently being developed for a variety of viral infections. Its currently undergoing clinical trials for COVID-19 in Brazil [27]. Dexamethasone is a common corticosteroid (steroid) medication that has been used for many years to treat various health conditions, such as autoimmune conditions and allergic reactions. Clinical trials are currently in the UK to investigate its effectiveness on COVID-19 [28,29].
Immunoglobin therapy
Antibodies from recovered patients may help with both free virus and infected cell immune clearance. This has been found to be effective for the H1N1 virus or swine flu. Previous studies on SARS and MERS, two related coronaviruses that had previously led to outbreak in people, have shown that the antibody levels for the new, SARS-COV-2 may drop much quickly [30]. The above synthetic antiviral agents, which when administered, can in addition to suppress the proliferation of the virus, cause irreversible side effects. Thus, there is a need to use alternative or complementary medicines such as plant extracts.
Plant Extracts as Antiviral Agents to Fight COVID-19
Plant extracts, in their crude state or via the isolation and purification of natural products have been used as antimicrobial [31-36]. Antidiabetic [37-41], anticancer agents [42-44]. Isolated natural products from plants have provided the platform for the design and synthesis of drugs in the Pharmaceutical industry. Over 50% of modern drugs are derived from natural products or derivatised natural products. There is also an increase use of plant extracts as medicinal therapeutics. However, truly lacking in antivirology studies is the use of plant extracts as antiviral agents. Research in this direction has been progressing in vivo and in vitro and needs to be intensified, in comparison to antimicrobial studies or other bioassays, considering the search for a COVID-19 vaccine or medication.
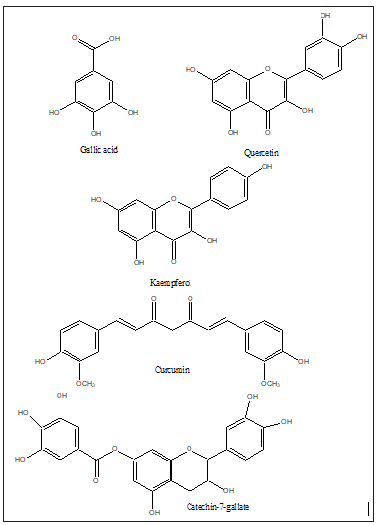
The same plant extracts can have different antiviral activity against RNA and DNA viruses. However, such research needs to be fast tract, considering the emergence of new viral strains such as which has been the cause of Covid-19 [45]. A wide range of natural products such as flavonoids, terpenoids, lignans, sulphides, polyphenolics, coumarins, saponins, furyl compounds, alkaloids, polyenes, thiophenes, proteins and peptides have been identified as possible antiviral agents [46-49]. In addition, selected essential oils of culinary herbs, spices and herbal teas have been shown to exhibit a significant level of antiviral activities [50,51]. Due to the high prevalence of viral infections, having no specific treatment and the constant appearance of resistant viral strains, the development of novel antiviral agents is essential. (Figures 10&11) show a list of natural products with promising antiviral activities and which should form the platform for other synthetic drugs mimicry (Figures 12-15).
Figure 10: Azithromycin.
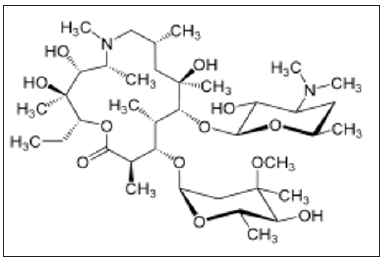
Figure 11: Tamiflu.
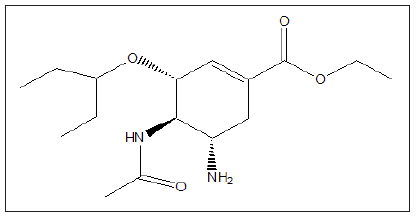
Figure 12: Colchine.
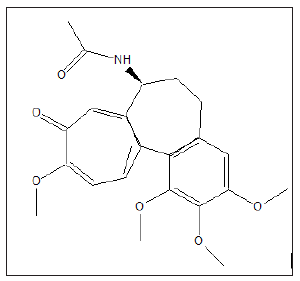
Figure 13:Ivermectin.
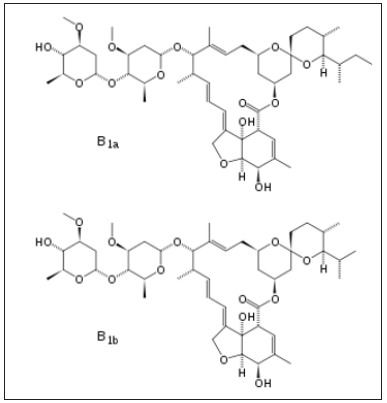
Figure 14:Galidesivir.
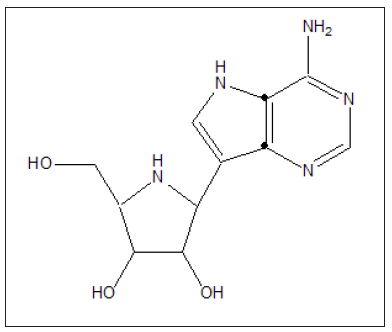
Figure 15:Dexamethasone.

Amongst the list of plants studied for their antiviral activity are Ricinus communis, Abrus precatorious, Adenia digitata, Rutaceae and Umbelliferae (Apiaceae), Canavalia ensiformis, lens culinaris, Phaseolus vulgaris, Triticum vulgaris, Amanoa aff. Oblongifolia, Juniperus communis, Justicia procumbens, Podophyllum peltatum, Kadsura matsudai, Aloe barbadensis, Aster scaber, Cassia angustifolia, Dianella longifolia, Euodia roxburghiana, Geum japonicum, Hamamelis virginiana etc. Asteraceae, Apiaceae, Campanulaceae Panax ginseng, Chrysanthemum sibiricum, Achyrocline flaccida, Bostrychia montagnei, Cedrela tubiflora, Prunella vulgaris, Sclerotium glucanicum, Stevia rebaudiana, Rhizophora mucronata, Ricinus communis, Abrus precatorius, Adenia digitata, Aspilia, Chenactis douglasii, Dyssodia anthemidifolia, Eclipta alba, Eriophyllum lanatum, Rutaceae, Camptotheca acuminate, Atropa belladona, Swainsona canescens, Ricinus communis, Abrus precatorious, Adenia digitata, Rutaceae, Camptotheca acuminate, Atropa belladona, Swainsona canescens, Ricinus communis, Abrus precatorious, Adenia digitata (Figures 16-18).
Figure 16:
A possible herbal cure for COVID-19 can be realized via the use of two or several of the above plants. Plants are said to possess a wide array of natural products and so present many structural conformations to the virus. Anti-viral drugs must present several conformations to the virus, since viruses can mutate and change conformation. Currently, it
Figure 17: Some natural products with promising antiviral activities.
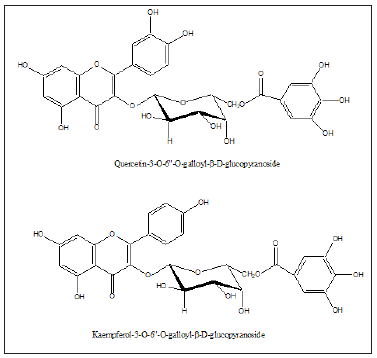
Figure 18: apigenin: 5, 7-dihydroxy-2-(4-hydroxylphenyl)- 4H –chromen-4-one, C15H10O5.
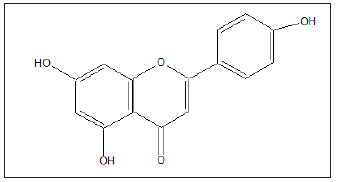
Conclusion
COVID-19, the current planet threatening disease, has indeed inflicted and affected humanities in all forms. There is a urgent need to find the correct drug combination or the requisite vaccine or correct herbal nontoxic mixtures to eradicate this disease. There is no known vaccine developed to date. Over 150 pharmaceutical companies are in pursuit of a cure. Its anticipated that before the end of 2020, the first COVID-19 vaccine would be available.
References
- (2020) Naming the coronavirus disease (COVID-19) and the virus that causes it. World Health Organization (WHO).
- (2020) Novel coronavirus, China. World Health Organization (WHO).
- Huang C, Wang Y, Li X, Ren L, Zhao J, et al. (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395(10223): 497-506.
- (2020) Statement on the second meeting of the international health regulations (2005) emergency committee regarding the outbreak of novel coronavirus (2019-nCoV). World Health Organization (WHO).
- (2020) WHO director-general's opening remarks at the media briefing on COVID-19-11 March 2020. World Health Organization.
- (2020) How COVID-19 spreads. US Centers for Disease Control and Prevention (CDC), USA.
- Bourouiba L (2020) Turbulent gas clouds and respiratory pathogen emissions: Potential implications for reducing transmission of COVID-19. JAMA.
- Trevor AJ, Katzung BG, Masters SB (1998) Pharmacology. In: McGraw-Hill (Ed.), Lange Medical Book. (7th edn), Medical Publishing Division.
- Madigan M, Martinko J (2006) Brock biology of microorganisms. In: (11th edn), Prentice Hall, USA.
- (2018) The lytic cycle of the t-even bacteriophage. Nemetoadreviews.com.
- Smith CM, Reynard AM (1992) Textbook of pharmacology. Saunders Company, USA.
- (2020) FDA cautions against use of hydroxychloroquine or chloroquine for COVID-19 outside of the hospital setting or a clinical trial due to risk of heart rhythm problems. U.S. Food and Drug Administration (FDA).
- (2020) FDA revokes emergency use of hydroxychloroquine. CNBC; "HCQ and CQ revocation letter. US Food and Drug Administration (FDA).
- (2020) Frequently asked questions on the revocation of the emergency use authorization for hydroxychloroquine sulfate and chloroquine phosphate. US Food and Drug Administration (FDA).
- (2020) List of references of observational studies of chloroquine and hydroxychloroquine in COVID-19 patients. European Medicines Agency (EMA).
- (2020) A trial of remdesivir in adults with severe COVID-19. ClinicalTrials.gov.
- (2020) A trial of remdesivir in adults with mild and moderate COVID-19. ClinicalTrials.gov.
- (2020) Study to evaluate the safety and antiviral activity of remdesivir (GS-5734) in participants with moderate coronavirus disease (covid-19) compared to standard of care treatment. ClinicalTrials.gov.
- Ng E (2020) Coronavirus: are cocktail therapies for flu and HIV the magic cure? South China Morning Post.
- Zheng W, Lau M (2020) China's health officials say priority is to stop mild coronavirus cases from getting worse. South China Morning Post, Hong Kong.
- Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, et al. (2020) Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents 56(1): 105949.
- Creary EK, Pogue JM (2020) Coronavirus disease 2019 treatment: A review of early and emerging options. Open Forum Infectious Diseases 7(4): ofaa105.
- (2020) Coronavirus COVID-19 (SARS-CoV-2). Johns Hopkins ABX Guide.
- (2020) New clinical study: Potential treatment for coronavirus will be tested in Canada as of today. Bio Space.
- (2020) TWiV 599: Coronavirus update, we need a plan | This Week in Virology. Bray Controls Limited, UK.
- Mike R, Craig N, François J, David W, Kylie (2020) Ivermectin and COVID-19: a report in antiviral research, widespread interest, an FDA warning, two letters to the editor and the authors' responses. Antiviral Res 178: 104805.
- (2020) A study to evaluate the safety, pharmacokinetics and antiviral effects of Galidesivir in yellow fever or COVID-19. ClinicalTrials.gov.
- (2020) Dexamethasone and COVID-19. Specialist Pharmacy Service.
- (2020) COVID-19 treatment guidelines. National Institutes of Health.
- Masihi KN (2001) Fighting infection using immunomodulatory agents. Expert Opin Biol Ther 1(4): 641-653.
- Shahid W, Durrani R, Iram S, Durrani M, Khan F (2013) Antibacterial activity in vitro of medicinal plants. Sky Journal of Microbiology Research 1(2): 5-21.
- Arif T, Bhosale JD, Kumar N, Mandal TK, Bendre RS, et al. (2009) Natural products-antifungal agents derived from plants. J Asian Nat Prod Res 11(7): 621-638.
- Aarati N, Ranganath N, Soumya G, Kishore B, Mithun K (2011) Evaluation of antibacterial and anticandidal efficiency of aqueous and alcoholic extract of neem (Azadirachta indica). International Journal of Research in Ayurveda & Pharmacy 2(1): 230-235.
- Jagessar RC, Mohamed N (2010) Antimicrobial activity of selected plants extracts from Guyana’s flora. Journal of Pure and Applied Microbiology 4(2): 533-540.
- Jagessar RC, Allen R (2011) Antimicrobial potency of the aqueous extract of leaves of terminalia CATAPPA. Academic Research International 1(3): 362-371.
- Jagessar RC, Mars A, Gomathigayam S (2011) Selective antimicrobial properties of Leaf extract of Samanea Saman against Candida albicans, Staphylococcus aureus and Escherichia coli using several microbial techniques. Journal of American Science 7(3): 108-119.
- Ngugi MP, Njagi JM, Kibiti CM, Miriti PM (2012) Pharmacological management of diabetes mellitus. Asian Journal of Biochemical and Pharmaceutical Research 2(2): 375-381.
- Najmi A, Akhtar M, Mohd A, Mujeeb M, Pillai KK, et al. (2012) A pharmacological appraisal of medicinal plants with antidiabetic potential. J Pharm Bioallied Sci 4(1): 27-42.
- Rai PK, Jaiswal D, Mehta S, Watal G (2009) Anti-hyperglycaemic potential of Psidium guajava raw fruit peel. Indian J Med Res 129(5): 561-565.
- Huang CS, Yin MC, Chiu LC (2011) Antihyperglycemic and antioxidant potential of Psidium guajava fruit in Streptozotozin induced diabetic rats. Food Chem Toxicol 49(9): 2189-2195.
- Banu MS, Sridharan SK, Manikandan R (2013) Antihyperglycemic and antihyperlipidemic potentials of Psidium guajava in alloxan-induced diabetic rats. Asian Journal of Pharmaceutical and Clinical Research 6(1): 88-89.
- Siriwantanmetanon N, Fiebich BL, Efferth T, Prieto JM, Heinrich M (2010) Traditionally used thai medicinal plants: In vitro anti-inflammtory, anticancer and antioxidant activities. J Ethnopharmacol 130(2): 196-207.
- Heo BG, Park YJ, Park YS, Bae JH, Cho JY, et al. (2014) Anticancer and antioxidant effects of extracts from different parts of indigo plant. Industrial Crops and Products 56: 9-16.
- Cao J, Xia X, Chen X, Xiao J, Wang Q (2013) Characterisation of flavonoids from Dryopteris erythrosora and evaluation of their antioxidant, anticancer and acetylcholinesterase inhibition activities. Food Chem Toxicol 51: 242-250.
- Solowey E, Lichtenstein M, Sallo S, Paavilainen H, Solowet E, et al. (2014) Evaluating medicinal plants for their anticancer activity. Scientific World Journal 1-12.
- Hui DS, Azhar E, Madani TA, Ntoumi F, Kock R, et al. (2020) The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health, the latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis 91: 264-266.
- Sakagami H, Sakagami T, Takeda M (1995) Antiviral properties of polyphenols. Polyphenol Actualities 12: 30-32.
- Gauntt CJ, Wood HJ, McDaniel HR, McAnalley BH (2000) Aloe polymannose enchances anti-coxsackievirus antibody titres in mice. Phytotherapy Research 14(4): 261-266.
- Bunyapraphatsara N, Dechsree S, Yoosook C, Herunsalee A, Panpisutchai Y (2000) Anti-herpes simplex virus component isolated from Maclura cochinchinensis. Phytomedicine 6(6): 421-424.
- Dai JR, Hallock YF, Cardellina JH, Boyd MR (1998) HIV-inhibitory and cytotoxic oligostilbenes from leaves of Hopea malibato. J Nat Prod 61(3): 351-353.
- Cox SD, Mann CM, Markham JL (2001) Interactions between components of the essential oil of Melaleuca alternifolia. J Appl Microbiol 91(3): 492-497.
© 2021 Jagessar RC. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






