- Submissions

Full Text
Novel Research in Sciences
Application of Lactic Acid Bacteria for Preand Post-Harvest Treatment to Control Listeria Monocytogeneson the Surface of Produce
Tong Zhao*, Govindaraj Dev Kumar and Minji Hur
College of Agricultural and Environmental Sciences, University of Georgia, Georgia
*Corresponding author: Djoko Susanto, Professor at YKPN School of Business, Indonesia
Submission: September 19, 2020;Published: September 28, 2020
.jpg)
Volume4 Issue5September, 2020
Abstract
Two lactic acid bacterial isolates, C-1-92 (Lactococcus lactis subsp. Lactis) and #152 (Enterococcus durans) with excellent inhibitory activity against L. monocytogenes at 4-37 oC in various conditions were evaluated. A cocktail of three L. monocytogenes strains were used to inoculate green peppers and apples at 21±1 oC at a concentration of 3log CFU/cm2. After 48h post-inoculation, green peppers and apples were individually treated with 0.1% peptone, 5log CFU/ml C-1-92 or #152. These two lactic acid bacteria were further evaluated in hot and green pepper plants by similar methods. After 14 days the population of L. monocytogenes on green pepper treated with control, #152, and C-1-92 studies was 3.4, 2.3, and 1.3log CFU/cm2, respectively. The application of C-1-92 treatment reduced the L. monocytogenes population by 2.1log CFU/cm2 (P<0.05) when compared to the control. For apple studies, L. monocytogenes population treated with C-1-92 or #152 were below detection level (0.69CFU/cm2, P<0.05) and 50% samples were detected by selective enrichment only at day 14; whereas, L. monocytogenes in control group was 2.6CFU/cm2. Pepper plants studies indicated that the treatment significantly reduced listeria count 5 days following treatment. The average count of listeria in C-1-92 or #152 treated hot peppers was 0.7 and 0.96log CFU/cm2, respectively, whereas the average count in hot peppers of control group was 2.5log CFU/cm2. Listeria reduction from green pepper plants were similar with results obtained from hot pepper plants study. The intervention by Lactococcus lactis subsp. Lactis (C-1-92) can substantially reduce the existence of L. monocytogenes on the surface of fresh produce.
Keywords: Lactic acid bacteria; Listeria monocytogenes; Produce
Introduction
Outbreaks of illness due to foodborne pathogens associated with fresh produce have increased in the past decade [1-6]. Fruits and vegetables contain rich carbohydrate, fibre, and various vitamins. Consumers prefer ready to eat (RTE) food, which fruit and vegetable can be consumed right out of the package without washing, to offer consumers high nutrition, convenience, and value. The contamination of L. monocytogenes is common in RTE food, especially fruits and vegetables. Recent recall issued by Food and Drug Administration involved nectarines, peaches, and plums involved a dozen states and many stores for potential L. monocytogenes contamination. These recalls gave farmers, processing companies, and retailers tremendous loss and bad reputation, and hurt consumer’s confidence for RTE food and bad influence for food industry. A recent outbreak of Listeria monocytogenes infections was identified to link to whole cantaloupe contamination, involved 28 States, illnesses to 147 persons with 143 hospitalization and 33 deaths. Its survival mechanisms were not characterized, an effective, practical, cost-effective, and environmental-friendly method for control of L. monocytogenes in fruits and vegetables is urgently needed [6-12].
monocytogenes is hard to eliminate in the produce processing environment. It can survive many years in the fresh produce processing environment, especially floor drains as a long-term resident. Some specific strains can keep in a dormant status even under strict sanitation condition. These dormant cells can recover and start to grow, even can form the biofilm at space in the processing facilities and their locations may be very hard to reach for regular sanitization processing. These tiny biofilms can accumulate millions of L. monocytogenes cells and can become the source of contamination, cross-contamination, and re-contamination in various produce products as long as they get the chance to detach and initiate attachment to food processing surface. The presence of L. monocytogenes in mixed biofilms in produce processing chains has great concern because of their risk for cross-contamination of other surfaces [1,3,11].
The survival ability of L. monocytogenes is related with strain specific, contamination level, moisture, temperature, and environmental condition. Accumulation of a large amount of L. monocytogenes in a particular contact surface is needed for food contamination in a modern food facility because food containing various gradients, lower water activity, and pH will inhibit the growth of L. monocytogenes even contaminated at lower level. To accumulate large amount of L. monocytogenes will require biofilm formation. Only in this condition, large amount of L. monocytogenes embedded in the form of mixed biofilm can be accumulated. Many of the microbial intervention strategies for produce involve the use of antimicrobial chemicals in rinses or washes; however, the efficacy of most chemical intervention treatments is reduced by the presence of organic matter. More effective antimicrobial treatments are desired that are practical, cost-effective and safe to use [4]. Currently, there is no available intervention procedure for reduction of foodborne pathogens in pre-harvest produce.
Our previous studies have identified a group of bacteria with strong inhibitory zone against the growth of L. monocytogenes [13-19]. These isolates were individually evaluated at 4, 8, 15, and 37 oC for their log reduction against the growth of L. monocytogenes by tube and biofilm assays and nine isolates were identified. Biochemical and genetic studies reveal that they belong to three bacterial species, including Enterococcus durans, Lactococcus lactis, subsp. lactis and Lactobacillus plantarum [16]. Two isolates, including one isolate of E. durans and one isolate of L. lactis, were validated by applying them in floor drains in a poultry processing plant and a Ready-To-Eat poultry processing plant. Results revealed that biological treatment can greatly (>4 log CFU/100cm2) reduce the number of listeria sp. cells in floor drains at 3 to 26 oC in which fresh poultry is processed [17]. This purpose of this study is to validate this bio-control method for substantial reduction of L. monocytogenes contamination on fresh produce at pre-and post-harvest stages and to observe various factors to interfere its efficiency.
Materials and Methods
Green Peppers and apples
Fresh green peppers (150±25g) and apples (unwaxed Gala, 260±20g) purchased from a local retailor. Peppers and apples selected randomly used for tests of food-borne pathogens (Listeria, E. coli, and Salmonella) and aerobic bacteria counts by the methods we reported previously [15].
Bacteria strains
A mixture of three Listeria monocytogenes strains (LM 101, serotype 4, salami isolate; H9666, serotype ½C, human isolate; and ATCC 5779 ½C, cheese isolate) were selected for inoculation study. Two isolates of lactic acid bacteria, including C-1-92 (Lactococcus lactis subsp. Lactis) and #152 (Enterococcus durans); one strain of L. innocua (produce isolate, lab collection) were used for the study. Each isolate was individually grown in 10ml Brain Heart Infusion medium (BHI, Becton Dickinson Microbiology Systems, Sparks, MD) for 16-18h at 37 oC. Cultures of the three well-grown L. monocytogenes strains containing approximately the same population were combined or individually Lactic acid bacteria, washed three times in a 50ml sterilized centrifuge tube in a centrifuge (Thermo, Milford, MA) at 4,000g for 20min, and suspended in 0.1M phosphate buffer, pH 7.2. The final suspension was adjusted to an optical density of 0.5 at 630nm 9 approximately 108 CFU/ml) as determined with a spectrophotometer (Spectronic instruments, Rochester, NY). Cell numbers were confirmed by plating 0.1ml of the suspension serially diluted (1:10) in 0.1% peptone water on tryptic soy agar (TSA, BD) and MOX (BD for listeria) MRS (BD for lactic acid bacteria). All plates were incubated at 37 oC for 24h.
Contamination of peppers and apples
Oneml of 3-strain L. monocytogenes mixture was washed for 3 times by 0.1% peptone by centrifugation at 4000g and pellet was suspended in 0.1% peptone water solution. The bacterial solution added to 1-L 0.1% peptone water to final concentration of 103CFU/ml in a 2-L glass beak with a magnetic bar at 150rpm. After mixed for 2minutes a volume of 300ml was transferred to a 500ml hand spray bottle. About 1.2±0.2ml was sprayed onto each green pepper or apple in a laminar hood and dried for 40min.
Preparation of treatment bacteria
Each treatment strain, Lactococcus lactis subsp. lactis (#C-1-92) and Enterococcus durans (#152), was grown individually in a 500ml Erlenmeyer flask containing 250ml Lactobacillus MRS broth (MRS, Becton Dickinson) at 32 °C for 24h. Cells were sedimented by centrifugation at 10,000xg for 20min at 4 °C. The bacteria were resuspended in 25ml of MRS broth at ca. 109CFU/ml, serially (1:10) diluted in 0.1% peptone, and plated on MRS agar and tryptic soy agar in duplicate for bacterial counts.
Treatment of peppers by lactic acid bacteria
The solution of C-1-92 (Lactococcus lactis subsp. Lactis) and #152 (Enterococcus durans) was adjusted to 105CFU/ml and sprayed on the surface of green pepper or apple (1.2±0.2-ml) and dried for 40min at a laminar hood. The peppers and apples were incubated at 21 oC and samples taken out for bacterial enumeration daily.
Enumeration of monocytogenes
Each bag with green pepper or apple with same amount of 0.1% peptone water (250ml) was vigorously rubbed by hands for 1min and shake in a horizontal shaker (Thermo Scientific) at 200 rpm for 2min. A volume of 1ml suspension from each bag was serially (1:10) diluted in 9ml 0.1% peptone up to 10-6 CFU/ml. A volume of 0.1ml from each diluted tube was surface plated on modified Oxoid agar (MOX, Oxoid) in duplicate for isolation of Listeria bacteria according to the methods described above and previously [18, 19].
Selective enrichment
When L. monocytogenes was not detected by direct plating, a Listeria selective broth was used, and a volume of 1ml was added to 9ml broth. The broth tubes were incubated for 24h at 37 °C. Following incubation, a 10-ml loopful from the broth tube was plated in duplicate onto MOX plates for Listeria, and plates were incubated for 24h at 37 °C. Colonies with typical Listeria morphology (black) were selected and transferred once more on MOX plates and incubated for 24h at 37 °C for further confirmation by genetic and immunological methods by the procedures described previously [16].
Determination of aerobic plate counts (APC), fecal coli-form, coli, and Salmonella
Serial dilutions of samples described above were surface plated on plate count agar plates (PCA, Becton Dickinson Microbiology Systems) and incubated at 30 °C for 72h for enumeration. For fecal coli-form, E. coli, and salmonella determination similar methods were selected according to the report we described previously [15].
Confirmation of monocytogenes
Colonies counted as Listeria sp. were randomly selected and transferred to MOX plates and confirmed as Listeria sp. by biochemical tests (API 20Iminiaturized diagnostic test) and lateral flow latex agglutination assay (Oxoid, Listeria Rapid Test), and as L. monocytogenes by an enzyme-linked fluorescent immunoassay and PCR assay [18].
Selection of pepper plants
Two kinds of pepper plants (hot pepper and green pepper) were selected for pre-harvest treatment studies. Pepper plants (4-6 weeks old) were purchased from a local retailor. These plants were grown for another 3 to 4 weeks at temperature ranged from 62 to 96 o F under direct sun. Then, they were individually transferred into a 3-gallon pot with all-purpose fertilizer to grow for another 3-5 weeks (height at 4 to 4.7 feet for hot pepper plants and 3.5 to 4 feet for green pepper plants) before experiment.
Contamination of Listeria
innocua (non-virulent Listeria isolate) was used for the contamination of pepper plants. The bacteria was grown in individual test tube containing 10ml of BHI media at 37 oC for 18h. Bacteria from 5 individual tube were combined in a 50ml centrifuge tube and washed by centrifuge at 4, 000rpm for three times. The pellet was suspended in 50ml 0.1% peptone water. Then, all of them were poured into a tank containing 600ml 0.1% peptone water. Under pressure the bacterial were surface-contaminated the pepper plants.
Treatment of pepper plants
Twenty-four hours after contamination of listeria, two lactic acid bacteria, including C-1-92 and #152 at similar concentration with the similar growth condition was individually sprayed on all pepper plants.
Collection of pepper
Peppers were individual collected by hand with sterile glove and placed in individual whirl-Pak bag (55oz, 1,627ml, 19 x 30cm, Nasco). A solution of 25ml of 0.1% peptone water was added into each bag and mixed with a shaker at 150rpm for 2min. The solution in each bag was serially (1:10) diluted to 10-6CFU/ml. The solution from each dilution tube was plated on the surface of MOX and PCA plates in duplicate. The plates were incubated at 37 oC for MOX or 30 oC for PCA at 48 to enumerate the listeria and aerobic bacteria according to the method described previously [15].
Statistical analysis
Statistical analysis was performed with SPSS 11.5 for Windows (SPSS Inc., Chicago, IL, USA). The data were presented as means ± standard error. Data were analyzed for analysis of variance (ANOVA) to determine significant difference (P<0.05) and for correlation coefficients (CORRELL) to determine the correlation between two group data.
Results
Figure 1: ABC, aerobic bacteria count.
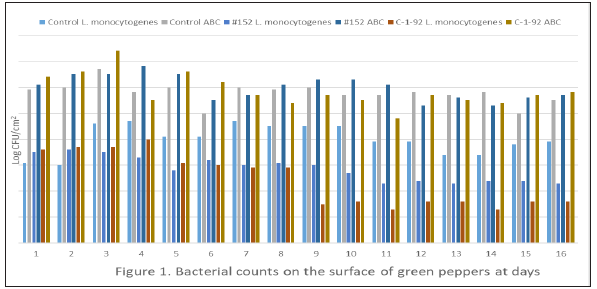
Figure 2: ABC, aerobic bacteria count.
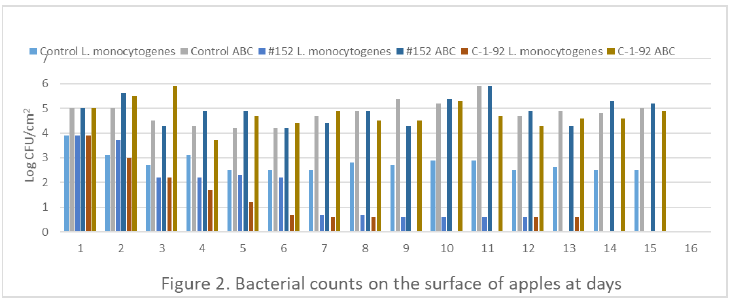
All peppers and apples were negative for isolation of Salmonella, E. coli O157:H7 and L. monocytogenes before experiment. The total aerobic bacteria counts were 4.7±0.4 for green peppers and 4.2±0.2 for apples. After 14 days, the population of L. monocytogenes on green pepper treated with 0.1% peptone water, Enterococcus durans, and Lactococcus lactis subsp. Lactis studies was 3.4, 2.3, and 1.3log CFU/cm2, respectively. The application of Lactococcus lactis subsp. Lactis treatment reduced the L. monocytogenes population by 2.1log CFU/cm2 (P<0.05) when compared to the peptone water only (Figure 1). For apple studies, L. monocytogenes population treated with Lactococcus lactis subsp. Lactis C-1-92 or Enterococcus durans were below detection level (0.69CFU/cm2, P<0.05) and 50% samples were detected by selective enrichment only at day 14; whereas, L. monocytogenes in control group was 2.6CFU/cm2 (Figure 2). The aerobic bacterial counts on peppers and apples revealed there were no difference among control and lactic acid-treated groups. The results demonstrated that the treatment by Lactococcus lactis subsp. Lactis (C-1-92) can substantially reduce the existence of L. monocytogenes on the surface of fresh produce.
The baseline determination of aerobic bacteria counts for hotter and green pepper before studies were 4.8±0.7 and 4.9±0.5, respectively. L. monocytogenes, Salmonella, and E. coli O157:H7 were not isolated from these pepper plants. Results obtained from pre-harvest treatment by Enterococcus durans or Lactococcus lactis subsp. Lactis on hot pepper plants demonstrated that application of both lactic acid significantly reduced the population of Lisetria on the surface of hot pepper plants. Starting from day 5, significant difference (P<0.05) of listeria count between control and treated hot pepper groups were demonstrated. The effect of treatment in either Lactococcus lactis subsp. Lactis or Enterococcus durans became more significant after 12 days. All samples collected from either treatment group turned to be negative for isolation of listeria. However, the positive isolation of listeria lasted to the end of the study (Table 1).
The aerobic bacteria count indicated that their population was increased at the first 5 days after the treatment was completed. However, this increase did not continuously increase, their number returned to their pre-treatment condition (104-105CFU/cm2) and kept this trend to the end of the study. Similar results were obtained with the green pepper plant study. Starting from day 6, the listeria counts from samples collected from either treated groups were reduced significantly (P<0.05, Table 2) when compared with samples collected from control group. This tendency was continuously lasted to the end of the study. As same as hot pepper results demonstrated. The counts of total aerobic bacteria were backed from the pre-treated condition (105 -106 CFU/cm2) after initial increase (Table 1).
Table 1: Bacterial counts on the surface of hot pepper plants. a (+) positive selective enrichment. b (-) negative selective enrichment.
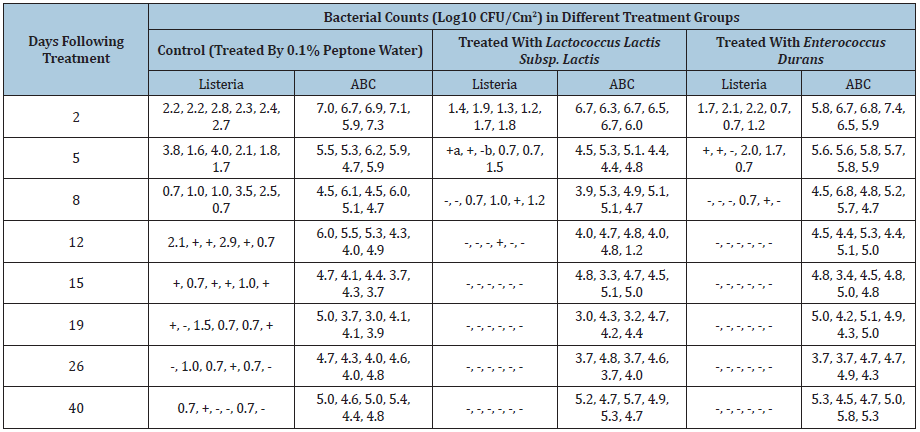
Table 2: Bacterial counts on the surface of green pepper plants. a (+) positive selective enrichment. b (-) negative selective enrichment.

Discussion
Our previous studies identified two lactic acid bacteria; they demonstrated strong ability to inhibit the growth of L. monocytogenes at various conditions with different temperatures. In this study, we individually evaluated its ability as a treatment for pre- and post-harvest fresh produce to reduce the listeria contamination. The comparison of listeria number and growth characteristics between pre-harvest and post-harvest stages indicated that the listeria was easier to survive at the surface of peppers and apples at post-harvest stage. Its survival capability might be related with surface temperature, humility, and nutrition [5]. At pre-harvest condition, surface temperature was high, especially under direct sunshine in the hot summer (>90 o F). The population of listeria reduced quickly, but lower number still could survive for a long time on the surface of hot or green pepper plants as shown in control groups (Table 1 & 2). This survived listeria may become the original source for cross-contamination during harvest management [1].
The advantages of traditional chemical method treatment include rapid reduction, cost-effective, and the best of all is chemical concentration can be continuously increased until effective elimination [13,14]. The disadvantage is contaminated listeria may form the biofilm at some parts of the produce and a quick wash may be hard to remove all of them. The L. monocytogenes can survive, reappear, and the biofilm becomes more solid than formed previously. This situation has been revealed that the same strain of L. monocytogenes is responsible for current outbreak and outbreak happened many years ago. L. monocytogenes can survive at the existing biofilms in produce processing environment, especially floor drains, and will be responsible for L. monocytogenes outbreaks. The application of biocontrol will ensure fresh produce from the listeria contamination before harvest and ensure processing facilities from the contamination of L. monocytogenes.
The purpose that we individually evaluate these two candidates is that produce include various products. Their chance to get the contamination is significantly various. Some of them grow on the surface of soil, such as, watermelon. Their chance to get the contamination of L. monocytogenes is much high. Thus, Enterococcus durans may be a better choice because their survivability is much strong in the bad environment [18]. However, some produce, such as, peppers, they grow on the plants and their chance to get the contamination of L. monocytogenes is low. Thus, the application of Lactococcus lactis subsp. Lactis should be a good selection because of its strong bacteriocin production [7].
The results of pre-harvest treatment indicated that its bactericidal effect could last more than four weeks from contamination by listeria suggested that time for application should start early, 4 to 6 weeks before harvest may be a good choice. From this study, bio-control application for once should be effective. All inoculated listeria was removed starting from week 2 and lasted for at least more than four weeks.
Conclusion
The application of this bio-control procedure demonstrates that they can substantially reduce the existence of L. monocytogenes on the surface of fresh produce at pre- and post-harvest stages.
Acknowledgement
This study was supported by a grant from Georgia Department of Agriculture.
References
- Bartz F, Lickness E, Heredia J, Aceituno N, Newman A, et al. (2017) Contamination of fresh produce by microbial indicators on farms and in packing facilities: Elucidation of environmental routes. Appl Environ Microbiol 83(11): 1-10.
- Bennett S, Littrell K, Hill T, Mahovic M, Behravesh C (2015) Multistate foodborne outbreaks associated with raw tomatoes, United States, 1990-2010: A recurring public health problem. Epidemiol Infect 143(7): 1352-1359.
- Bennett S, Sodha S, Ayers T, Lynch M, Gould L, et al. (2018) Produce-associated foodborne disease outbreaks, USA, 1998-2013. Epidemiol Infect 146(11): 1397-1406.
- Coelho M, Silva C, Ribeiro S, Dapkevicius M, Rosa H (2014) Control of Listeria monocytogenes in fresh cheese using protective lactic acid bacteria. Int J Food Microbiol 191: 53-59.
- Deblais L, Helmy Y, Testen A, Vrisman C, Madrid A, et al. (2019) Specific environmental temperature and relative humidity conditions and grafting affect the persistence and dissemination of Salmonella enterica Enterica serotype tymphimurium in tomato plant tissues. Appl Environ Microbiol 85(11): 1-17.
- Dias J, Simbras B, Beres C, Santos K, Cabral L, et al. (2018) Acid lactic bacteria as a bio-preservant for grape pomace beverage. Frontiers in Sustainable Food Systems 2: 1-8.
- Du L, Liu F, Zhao T, Zhao P, Doyle M (2017) Characterization of Enterococcus durans 152 bacteriocins and their inhibition of Listeria monocytogenes in ham. Food Microbiol 68: 97-103.
- Gómez N, Ramiro J, Quecan B, Franco B (2020) Use of potential probiotic lactic acid bacteria (LAB) biofilms for the control of Listeria monocytogenes, salmonella typhimurium, and Escherichia coli O157: H7 biofilms formation. Front Microbial 7: 1-15.
- Guerrieri E, Niederhãusern S, Messi P, Sabia C, Iseppi R, et al. (2009) Use of lactic acid bacteria (LAB) biofilms for the control of Listeria monocytogenes in a small-scale model. Food Control 20(9): 861-865.
- Hossain M, Mizan M, Ashrafudoulla M, Nahar S, Joo HJ, et al. (2020) Inhibitory effects of probiotic potential lactic acid bacteria isolated from kimchi against Listeria monocytogenes biofilm on lettuce, stainless-steel surfaces, and MBEC biofilm device. LWT 118: 108864.
- Kumar G, Williams R, Qublan AH, Sriranganathan N, Boyer R, et al. (2017) Airborne soil particulates as vehicles for Salmonella contamination of tomatoes. Int J Food Microbial 243: 90-95.
- Lim J, Lee C, Kim G, Bang Y, Rhim J, et al. (2020) Using lactic acid bacteria and packaging with grapefruit seed extract for controlling Listeria monocytogenes growth in fresh soft cheese. J Dairy Sci 103(10): 8761-8770.
- Olszewska M, Zhao T, Doyle M (2016) Inactivation and induction of sublethal injury of Listeria monocytogenes in biofilm treated with various sanitizers. Food Control 70: 371-379.
- Ramos T, Russell MJ, Millner P, Shade J, Misiewicz T, et al. (2019) Assessment of biological soil amendments of animal origin use, research needs, and extension opportunities in organic production. Frontiers in Sustainable Food Systems 3: 1-11.
- Zhao T, Ji P, Kumar G (2020) Pre-harvest treatment for reduction of foodborne pathogens and microbial load on tomatoes. Food Control 119: 107469.
- Zhao T, Doyle M, Zhao P (2004) Control of Listeria monocytogenes in a biofilm by competitive inhibition bacteria. Appl Environ Microbial 70(7): 3996-4003.
- Zhao T, Podtburg T, Zhao P, Schmidt B, Baker D, et al. (2006) Control of Listeria monocytogenes by competitive exclusion bacteria in floor drains of a poultry processing plant. Appl Environ Microbiol 72(5): 3314-3320.
- Zhao T, Podtburg T, Zhao P, Chen D, Baker A, et al. (2013) Reduction by competitive bacteria of Listeria monocytogenes in biofilms and Listeria bacteria in floor drains in a ready-to-eat poultry processing plant. J Food Prot 76(4): 601-607.
- Zhao T (2016) Biofilm formation of foodborne pathogens and their control in food processing facilities. Journal of Food: Microbiology, Safety & Hygiene 1(2): 1-3.
© 2020 Tong Zhao. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






