- Submissions

Full Text
Novel Research in Sciences
PRP and Autologous Tissue Regeneration from Fibroblast and Adipose Stem Cell
Ghislaine Beilin MD*
Past President of ESAAM, France
*Corresponding author: Ghislaine Beilin MD, Past President of ESAAM, European Society of Anti-Aging and regenerative Medicine, France
Submission: April 28, 2020;Published: May 18, 2020
.jpg)
Volume3 Issue5May, 2020
Opinion
Tissue regeneration has always been a big challenge in surgery and regenerative medicine. Platelet-Rich Plasma (PRP) has been used since many years in the field of dermatology [1- 4]. Clinical evidences have been reported in many fields such as wound healing, burned, fat transplantation and alopecia.
PRP is defined as a volume of autologous plasma that has a platelet concentration above baseline (i.e. above value in whole blood) [5]. As such, PRP contains not only a high concentration of platelets but also the full content of plasma clotting factors, the latter of which typically remain at their physiologic levels [6].
Before proving clinical efficacy of PRP, we have to focus on in-vitro evidences
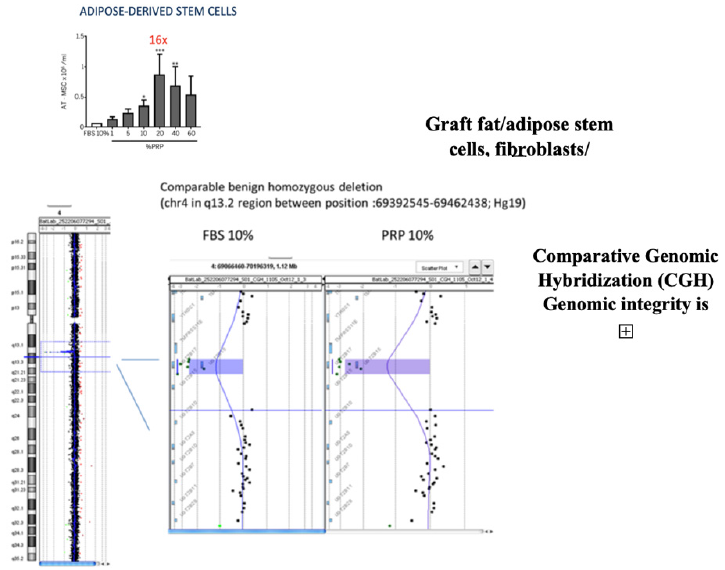
Berndt et al. [7] reported that PRP could enhance human fibroblast proliferation in a dose-dependent way. Indeed, in this study, a comparison between Fetal Bovine Serum (FBS) and non-activated PRP as a media for human fibroblast growth was performed and showed a significant increase (11x) in the proliferation rate in the PRP group compared to the FBS group. The adipose-derived stem cells proliferation increased (16X); Among this, a comparative genomic hybridization array was performed in order to ensure that the increased proliferation rate due to the PRP did not induce any genomic instability.
The scientific publication from a Swiss laboratory research team - Sarah Berndt, Antoine Turzi, Brigitte Pittet-Cuénod, and Ali Modarressi from the Geneva University Hospital (HUG) - was selected for the cover of the November 2019 issue of the prestigious Tissue Engineering journal. “Autologous Platelet-Rich Plasma (CuteCell PRP) Safely Boosts In Vitro Human Fibroblast Expansion (Figure 1)
Figure 1:

Figure 2:

This is the first-time a study demonstrates that specific cell extracts grow much faster in medium supplemented with Cutecell® PRP than with Fetal Bovine Serum (FBS), e.g. 10 times faster with fibroblasts and 16 times faster with adipose-derived stem cells (Figure 2).
Growth of cells in Cutecell® PRP did not affect their genomic structure, nor did it affect their phenotype, as is the case [contrary to what is observed] when cells are cultured in FBS.
Moving to clinical evidences, PRP has shown his effectiveness in the field of wound management for treating hard-to-heal or non-healing wounds. From a clinical point of view, the beneficial effect of PRP for the healing of chronic wounds such as legs ulcers, diabetic ulcers, pressures sores [8,9] or acute wounds such as splitthickness skin graft, surgical wounds, fistulas, burns [10-13] has been evidenced in a high number of clinical investigations (Figure 3).
Figure 3:
In chronic wounds, PRP treatment generally shows positive outcomes with no complication during the procedure or the post-treatment period. Interestingly, PRP is also able to provide a beneficial effect when there is no other therapeutic option for the treatment of a refractory wound. In general, complete closure can be obtained after several weekly topical applications and/or injections in the wound bed [14].
For acute wounds, PRP treatment has also shown to be effective for a variety of wounds, especially when this cannot be achieved with conventional therapies. In addition, it was shown to reduce post-operative pain, healing time, and the occurrence of infections compared to alternative treatments.
For example, in cardio-vascular surgery, PRP is able to significantly reduce the incidence of sternal wound infections [15] or treat driveline infections [16]. On the other hand, regarding the use of PRP combined with fat tissue; we know that autotransplantation of adipose tissue is commonly used for the treatment of tissue defects in plastic and reconstructive surgeries.
The reduced survival of a large percentage of the transplanted adipose tissue remains an unsolved issue. This is at least in part due to accelerated apoptosis of the implanted pre-adipocytes [17].
Platelet-released growth factors stimulate angiogenesis, cell differentiation and proliferation leading to reconstitution of the 3-D matrix that allows the rearrangement of adipocytes into the correct 3-D organization [18]. In vitro studies have demonstrated that PRP can significantly induce the proliferation of adipose tissue-derived stem cells [19].
Another innovation in terms of combination was the mixture between Hyaluronic Acid and PRP directly in one-step (Cellular Matrix technology, RegenLab, Le Mont-Sur-Lausanne, CH) for the use of skin aging management. Hersant and Méningaud [20] investigated the effects on skin firmness and elasticity of the PRP/ HA combination obtained with CellularMatrix (CM-PRP-HA). Thirty-one patients were treated with three injections one month apart using the mesotherapy injection technique. Patients’ skin was assessed at baseline and over a 6-month follow-up period using both objective parameters with a Cutometer and subjective criteria using the FACE-Q scale. Significant improvement between baseline and 6 months was observed for both the FACE-Q scores and the biophysical measurements obtained with the Cutometer, with no serious adverse reaction. All these clinical outcomes reveal the implication of PRP in different pathologies and its effectiveness in the skin aging process.
There is a new trend in cell therapies confirmed by the creation of a biologic laboratory launched in Carlsbad (California) by Dr Bert Mandelbaum, the famous KOL in sport medicine who just announced the creation of the Regenerative Institute in the Cedars- Sinai Medical Center. One project is the evaluation of RegenPRP® in patients and in parallel in the laboratory, e.g. cell counts, growth factors and other protein characterization, for the understanding of cartilage regeneration in order to create a unique cell-patient database.
This is a great breakthrough in tissue engineering, opening multiples clinical application: from wound healing ,graft transplant to healing process in diabetic ulcer. But also in in regenerative medicine: from musculo squeletic, osteo-tendinopaty or dermocosmetic and skin care to genital rejuvenation and In vitro fertilization with the preservation of genome integrity. In The current context, studies are also underway to evaluate the prophylactic or therapeutic effect of human plasma healed from Covid-19.
References
- Abuaf OK, Yildiz H, Baloglu H, Bilgili ME, Simsek HA, et al. (2016) Histologic Evidence of New Collagen Formulation Using Platelet Rich Plasma in Skin Rejuvenation: A Prospective Controlled Clinical Study. Ann Dermatol 28(6): 718-724.
- Cho JM, Lee YH, Baek RM, Lee SW (2010) Effect of platelet-rich plasma on ultraviolet b-induced skin wrinkles in nude mice. J Plast Reconstr Aesthet Surg 64(2): e31-39.
- Cho JW, Kim SA, Lee KS (2012) Platelet-rich plasma induces increased expression of G1 cell cycle regulators, type I collagen, and matrix metalloproteinase-1 in human skin fibroblasts. Int J Mol Med 29(1): 32-36.
- Kim DH, Je YJ, Kim CD, Lee YH, seo YJ, et al. (2011) Can platelet-rich plasma be used for skin rejuvenation? Evaluation od effects of platelet-rich plasma on human dermal fibroblast. Ann Dermatol 23(4): 424-431.
- Marx RE. (2001) Platelet-rich plasma what is PRP and what is not PRP. Implant Dent 10(4): 225-228.
- Eppley BL, Pietrzak WS, Blanton M (2006) Platelet-rich plasma: a review of biology and applications in plastic surgery. Plast Reconstr Surg 118(6): 147e-159e.
- Berndt S, Turzi A, Pittet-Cuénod B, Modaressi A (2019) Autologous Platelet-Rich Plasma (CuteCell PRP) Safely Boosts In Vitro Human Fibroblast Expansion. Tissue Engineering Part A 25 :21-22.
- Monami M, Mirabella C, Scatena A, Nreu B (2017) CO2 laser for the treatment of diabetic foot ulcers with exposed bone. A consecutive series of type 2 diabetic patients. Journal of Endocrinol Invest 40(8): 819-822.
- Kontopodis N, Tavlas E, Papadopoulos G (2016) Effectiveness of Platelet-Rich Plasma to Enhance Healing of Diabetic Foot Ulcers in Patients With Concomitant Peripheral Arterial Disease and Critical Limb Ischemia. Int J Low Extrem Wounds 15(1): 45-51.
- Guerid S, Darwiche SE, Berger MM, Applegate LA, Benathan M, et al. (2013) Autologous keratinocyte suspension in platelet concentrate accelerates and enhances wound healing - a prospective randomized clinical trial on skin graft donor sites: platelet concentrate and keratinocytes on donor sites. Fibrogenesis & tissue repair 6(1): 8.
- Filomia D, Ventura C, Crescibene A, Almolla J, Napolitano M (2013) Treatment of pilonidal sinus disease with autologous platelet-rich plasma. G Ital Dermatol Venereol 148(11): 704-706.
- Nicoli F, Balzani A, Lazzeri D (2015) Severe hidradenitis suppurativa treatment using platelet-rich plasma gel and Hyalomatrix. Int Wound J 12(3): 338-344.
- Teodoreanu RN, Popescu SA, Lascar I, Vulturescu V, Grigore A (2014) Therapeutic protocol using growth factors in electrocution ounds--case reports and review of the literature. Rom J Morphol Embryol 55(2): 473-482.
- Cervelli V, Lucarini L, Spallone D, Brinci L, de Angelis B
(2010) Use of platelet rich plasma and hyaluronic acid on exposed tendons of the foot and ankle. J Wound Care: 186: 8- 90. - Serraino GF, Dominijanni A, Jiritano F (2015) Platelet-rich plasma inside the sternotomy wound reduces the incidence of sternal wound infections. Int Wound J 12: 260-264.
- Jiritano F, Serraino GF, Cristodoro L, Renzulli A (2013) Ventricular assist device abdominal driveline infection: treatment with platelet-rich plasma. J Thorac Cardiovasc Surg 145(6): e69-e70.
- D’Esposito V, Passaretti V, Perruolo G, Ambrosio MR, Valentino R, et al. (2014) Platelet-rich plasma increases growth and motility of adipose tissue-derived mesenchymal stem cells and controls adipocyte secretory function. J Cell Biochem 116(10): 2408-2418.
- Modaressi A (2013) Platelet Rich Plasma (PRP) Improves Fat Grafting Outcomes. World J Plast Surg 2(1): 6-13.
- Atashi F, Jaconi ME, Pittet-Cuénod B, Modarressi A (2015) Autologous platelet-rich plasma: a biological supplement to enhance adipose-derived mesenchymal stem cell expansion. Tissue engineering Part C, Methods 21(3): 253-262.
- Hersant B, SidAhmed-Mezi M, Niddam J, La Padula S, Noel W, et al. (2015) Efficacy of autologous platelet-rich plasma combined with hyaluronic acid on skin facial rejuvenation: a prospective study. J Am Acad Dermatol 77(3): 584-586.
© 2020 Ghislaine Beilin MD. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






