- Submissions

Full Text
Novel Research in Sciences
Collateral Circulation and Secondary Hypertension Response in Adult Yucatan Minipigs after Experimental Aortic Coarctation
Cusack PTE*
Canada
*Corresponding author:Maria Aguirre- Sanceledonio, Department of Small Animal Clinical Sciences and Veterinary Pathology, Las Palmas de Gran Canaria, Spain
Submission: December 16, 2019;Published: January 16, 2020
.jpg)
Volume3 Issue1January, 2020
Abstract
Coarctation of the thoracic aorta was produced in 9 adult Yucatan mini pigs. A gradually aortic constriction was accomplished by placing a “C” shaped expandable occluder around the thoracic aorta. The aortic constriction was standardized by measuring the blood pressure above the coarctation with 165-170mm Hg mean arterial pressure as the target. The gradual occlusion of the aorta was performed over a period of 15 days. The pigs were studied for 4 (n=3) and 8 (n=5) weeks of cranial hypertension and then euthanized. Three animals served as controls. There were no deaths associated with placement of the occluder or constriction of the aorta and neither rear limb weakness nor neurologic dysfunction were noted. Aortic angiography demonstrated a severe grade of stenosis and extensive collateral circulation in the 8-week study pigs. This study showed that gradual coarctation of the thoracic aorta in adults and mature minipigs produces chronic and cranial hypertension and is associated with development of an extensive collateral circulation after 8 weeks of the study. The purpose of this work is to reveal the collateral growth and vascular patterns developed around the coarctation. To our knowledge, this collateral circulation response has not previously shown under these conditions and proofs that adult porcine individuals can adjust their vasculature to stenotic arterial condition showing dramatical vascular changes in a relatively short period of time.
Keywords:Aortic coarctation; Minipig, Hypertension; Collateral circulation; Angiography
Introduction
Coarctation of the aorta is a common cardiovascular malformation accounting for 5% to 8% of patients with congenital heart disease [1]. One out of every few thousand babies are born with a constricted aorta at a point beyond the arterial branches to the head and arms, but proximal to the kidney. This condition is known by coarctation of the aorta [2]. Elevation of the blood pressure, as well as the unusual distribution of this elevation, produces a unique physiological disturbance. The arterial pressure in the upper part of the body (cranial to the occlusion [prestenotic aorta]) usually averages from 20 to 55 per cent higher than in the lower part of the body or caudal to the occlusion (poststenotic aorta) [2,3].With coarctation, much of the blood flow to the lower body is carried by collateral blood vessels in the body wall [3]. These vessels form to help compensate for the abnormally high vascular resistance between the upper aorta and the lower aorta. The various mechanisms involved in the pathogenesis of this type of hypertension, although important in the understanding the physiologic alterations in aortic coarctation, are unclear [2,4]. The mechanism of the upper body or prestenotic hypertension in coarctation can, in part, be explained by mechanical factors, the role played by the kidneys (one kidney Goldblatt model) and vascular wall remodeling of the arterial network [5]. Although, there is much to be gained from furthering our understanding of the causes and effects of hypertension on the cardiovascular system. Different classes of animal models of hypertension have been described in the literature. Most of them are developed in rats and present easy management and a modest cost. However, the rat can tolerate 100% of acute aortic coarctation without clinical signs and its vascular response to hypertension and collateral circulation development differ notably when it is compared with human being and large animal vasculature. In contrast, the porcine vasculature is perhaps the closest to the human [6], and although experimental aortic coarctation has been produced in pigs before, it has been based in acute hypertension studies under anesthesia or the animals were partially coarctated when they were very young.
In the present study, we evaluate the development of chronic hypertension and collateral circulation in conscious and adult pigs during gradual and controlled aortic coarctation. The aim of our study is to reveal the extensive collateral growth occurred around the coarctation and show the increases in both lumen size and number of anastomoses between cranial and caudal intercostals arteries close to the occluder site at 8 weeks of occlusion. These neoformed vascular channels showed vascular patterns consistent with angiogenesis and arteriogenesis on both sides of the occlusion and reveals the collateral circulation as a long-term adaptation to the schemic flow.
Materials and Methods
Animal model
Animal care in this study conformed to the guidelines of the University Laboratory Animal Care Committee of Texas A&M University. Coarctation of the aorta was induced in 9 mature (8-12-month-old) male Yucatan minipigs (Panapinto Micro Minipigs®; Masonville, CO, USA) (nos 1-9), weighing between 25 and 41kg. The pigs, which were kept in separate cages, were allowed to become acclimatized to the experimental environment for a minimum of 15 days before any intervention. All the animals were fed daily with 1lb of LabDiet® Mini-pig HF Grower Granulated (PMI Nutrition International, Inc, St Louis, MO, USA), divided into three feeds. Pigs were restrained in a sling and then mask induced with isoflurane and intratracheal intubation was performed for maintenance of anesthesia and for ventilation support during the surgery. The approach to the caudal thoracic aorta was made through the eighth intercostal space from a left lateral thoracotomy. The vascular occluder was placed around the aorta over a previous Gortex banding and the ends were sutured together with nonabsorbable material. The tubing of the occluder was passed through the chest wall to the dorso-caudal area of the neck, where a titanium subcutaneous vascular access port (VAP) (TI-VAP; Access Technologíes, Skokie, IL, USA) had been placed.
Occlusion and blood pressure monitoring
Aortic occlusion was started one week after surgery. Blood pressures above the coarctation were measured using two different invasive methods: a carotid vascular catheter attached to a vascular access port (VAP) inserted in 3 animals and a PROPAQ 106 Vital Signs Monitor (Protocol Systems Inc. Beaverton, OR, USA). In 6 pigs, a telemetric method (Data Sciences Inc., MN, USA, model TA111PA-D70) was used to record blood pressure and the telemetry unit catheter was tunnelled subcutaneously to the internal thoracic artery and positioned in the vessel distal to its origen at the aorta. Before occlusion was started, a baseline blood pressure was recorded from each animal. Then, variable amounts (0.3-0.5ml) of 50% dextrose were added to the occluder port. This operation was repeated every other day until target pressure was reached. Thereafter, blood pressure averages were taken every day for the telemetry animals and twice a week for the carotid VAP group. Target day was considered day 1 of the hypertension study and data was registered during 8 weeks.
Renin activity
Plasma renin activity (PRA) was determined from plasma samples taken from normotensive (control) minipigs weekly, and from hypertensive minipigs every other day for the first 2 weeks, then once weekly. PRA was measured on 1.0ml aliquots of EDTAtreated plasma using the GammaCoat Plasma Renin Activity RIA Kit, supplied by DiaSorin, Inc., Stillwater, MN. In this assay, an ACE inhibitor (phenylmethylsulfonyl fluoride) is added to plasma and the rate of angiotensin I synthesized over time by renin is measured using a competitive RIA with 125I-labeled angiotensin I and antiangiotensin I antibody coated test tubes. In-house tests of assay precision have shown within run and between run coefficients of variation of less than 10% and angiotensin I recovery of approximately 100% at physiologic levels of activity. The antibody coated test tube method has a minimal sensitivity to angiotensin I levels down to 18.0pg/tube which, based on preliminary experiments, is well below the level of sensitivity necessary to asses PRA in swine plasma.
Angiographic studies
After placement of the vascular occluder, control aortic angiograms were performed before the occlusion was initiated. The aortic angiograms (lateral and dorso-ventral) were made using a fluoroscope with a programmed phototimer (RTP9211G-PG, 1990, Toshiba Medical Systems, Inc, Tustin, CA, USA). A 7 F catheter was placed in the left carotid and 20ml of contrast was injected in each projection with a multiple injector (Medrad Mark V Plus, manufactured by Medrad UK, Ltd). After 8 weeks of hypertension, a second aortic angiogram was performed in 5 animals of the study to reveal and evaluate the presence and size of collateral arteries. Euthanasia was delayed for two days later to allow the contrast agent to be cleared. A complete necropsy was performed.
Control study
Three pigs served as controls for blood pressure measurements, renin activity and collateral circulation development. In these pigs, blood pressure was measured by inserting a carotid (VAP) in one pig and telemetry units in the other two animals. Blood samples were taken through a vascular access port. None of these animals underwent a thoracotomy for placement of an aortic occluder.
Statistical methods
The results of the systolic, diastolic and mean blood pressure were expressed as mean±SD. Desviation from normal distribution was evaluated by Kolmogorov- Smirnov statistic test. The followup data was divided in four different moments of the research experience to compare blood pressures between hypertensive pigs: Surgery day, 1st target day (Mean Arterial Blood Pressure ≥150mmHg), after 4 weeks of hypertension (n=3) and after 8 weeks of hypertension (n=5). Comparison of the evolution of these different measurements between hypertensive animals was made by paired Student’s t test to analyze the differences between the mean values in paired samples. Comparisons of the mean values between hypertensive animals and control animals in the same chronological moments were used by unpaired Student’s t test for independent samples. Statistical significance was taken as a p value < 0.05. Data analysis was performed using the SPSS Statistical Package (version 10.0 for Windows).
Results
Hypertension study and plasma renin activity
Coarctation of the middle thoracic aorta was successfully performed on 9 animals of the study, all of whom developed hypertension (Figure 1). The mean blood pressure was maintained at greater than 165mmHg during the 4 and 8-week study period. Target blood pressure in all pigs was reached between 17-39 days from the initial occlusion. Arterial blood pressures and the heart rate were easily recorded throughout the study and the pigs showed no signs of ischemic paralysis during the development of hypertension. Blood pressure could be analyzed by the Student’s t test for paired samples between the mean values of hypertensive pigs. Four moments of the experience were taken into consideration: Surgery day, 1st target day, after 4 weeks of hypertension and after 8 weeks hypertension. The mean values of mean arterial blood pressure (MAP) in paired samples are summarized in Table 1. Systolic and diastolic blood pressures evolution followed a similar pattern to MAP. Surgery day mean values of blood pressures, n=8, 146±12/109±11mmHg were significantly lower to 1st target day mean values, n=8 (174±17/126±14)mmHg, also to 4 weeks mean values, n=8 174±17/126±14 mmHg and to 8 weeks mean values, n=5 200±30/160±13mmHg, p<0.05. Mean values from 1st target day were significantly lower comparing with 4 (n=8) and 8 (n=5) weeks of hypertension, p<0.05. Finally, there were no significant differences between 4 (n=5) and 8 (n=5) weeks of hypertension, p>0.05.
Figure 1:Mean arterial blood pressures in five 8-weeks hypertensive pigs compared with two control pigs’ study (dashed lines).
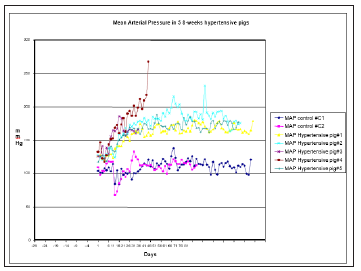
Table 1:Evolution of map in hypertensive pigs along the experience.
Comparison of the mean values between hypertensive pigs and controls were taken into consideration by unpaired Student´s t test for independent samples. This test showed that there were significant differences in the mean values of surgery day, 1st target day, and 4 weeks of hypertension between the hypertensive animals and the controls at the same chronological references, p< 0.05 (Table 2). Mean values of the controls at surgery day were n=3 122±12/94±11mmHg, at 1st target day were n=3 110±32/86±14 mmHg and at 4 weeks of hypertension were n=3 132±11/99±15mmHg. Hypertension was reversed in a pig of the study, the pre-occlusion average arterial pressures of 143±16/111±6 (127±7)mmHg increased to 175±9/158±10 (164±8) after target pressure were reached until day 20 of the study when arterial blood pressure decreased to 125±23/107±26 (114±24)mmHg. Additional pressure on the occluder was not succesful in increasing the pressure, and the occlusion was completely removed on day 21 of the study. The pressure increased slightly after removal of the aortic occlusion (171mmHg on day 25) and then decreased to lower than normal values before stabilized. The mean PRA from normotensive controls was 0.48±0.25±g/ml/hr over a 30- day period. In hypertensive minipigs, the PRA increased 5-fold or more within 5 days of the first occlusion of the aorta. Within 30 days of reaching target blood pressure of 150mmHg or more the PRA had returned to pre-occlusion levels, regardless of whether or not further inflation of the occluder and further constriction of the aorta occurred.
Table 2:MAP study: Comparisons between hypertensive and control group.

*Student’s t test between the mean values for independent samples.
Angiographic study
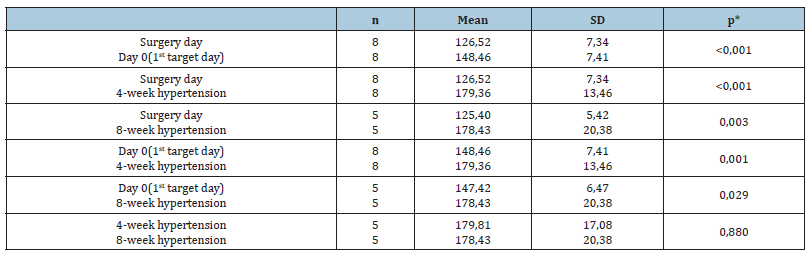
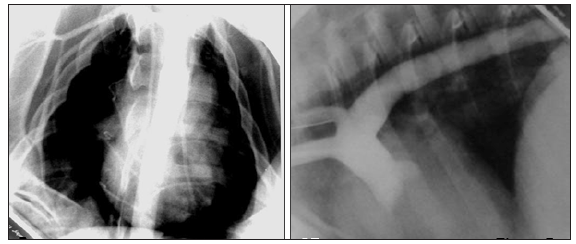
A clear and well-developed collateral circulation was observed in all the animals of the angiographic study after 8 weeks of reported hypertension (n=5), as was the effectiveness of the occluder to produce a complete obstruction of the thoracic aorta in an adult model (Figure 2) and (Figure 3). This extensive collateral circulation involved the intercostal arteries and their anastomoses cranial and caudal to the occlusion showing the characteristic corkscrew shape because of their circuitous course. These plexuses of curly arteries spanned through the intercostals muscles and the muscular collateral arteries network between the proximal aorta and distal aorta were clearly identified in the angiograms and were always compared with the control images (Figure 4) as normal references. The intercostal arteries were more tortuous and appeared to have an increased lumen and length compared with controls. Some other images revealed the presence of hypertrophied intercostal archs coming from the internal thoracic artery and anastomosing with dorsal intercostal arteries suppling blood flow to the caudal part of the aorta. A different process of collateralization could also be shown on the angiograms, where it appeared that the vasa vasorum of the aorta cranial to the occluder arose directly from the lumen of the aorta and bypassed the occlusion downstream from the lesion. In addition, we have observed small vascular channels running along the aorta wall, the pleura and mediastinum area towards the diaphragm and caudally to the occlusion of the aorta (Figure 2) and (Figure 3). At necropsy, the animals showed a significant enlargement of the dorsal intercostal arteries craneal and caudally to the occluder compared with the controls. There were also important changes observed in the adventitia vessels of the aorta wall of the hypertensive pigs which appeared more noticeable and pronounced.
Figure 2:Aortic angiograms that show extensive collateral circulation where intercostal arteries are clearly involved and their anastomosis through muscular collateral arteries network are also appreciated. Notice the extremely severe grade of aortic stenosis. CR: Cranial; R: Right
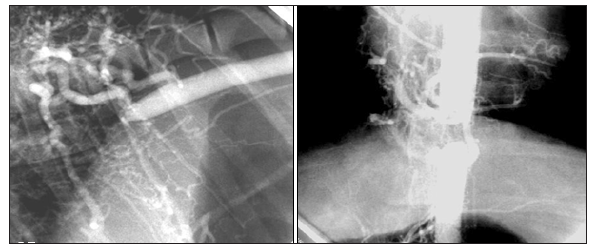
Figure 3:Fluoroscopic angiographic study in lateral projection shows sequential angiograms images. Notice the delay of the contrast re-entrance after coarctation site and the severe grade of aortic stenosis. CR: Cranial
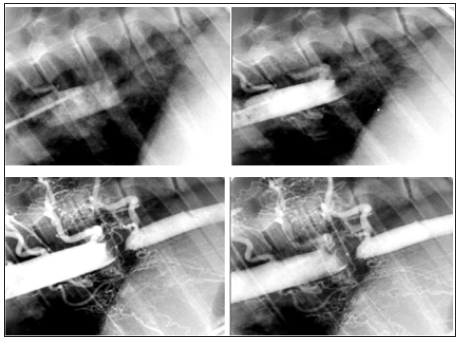
Figure 4:Angiograms control. Lateral and dorso-ventral projection respectively. CR: Cranial; R: Right
Discussion
Hypertension in aortic coarctation
All 9 animals under aortic coarctation showed a significant cranial hypertension during 4 or 8 weeks when compared with the control group. All hypertensive animals developed a rise in the systolic, mean, and diastolic blood pressures above the constriction which resembles the hypertension occurring in humans with coarctation of the aorta [1,7]. There was also a pronounced increase in the diastolic phase of the blood pressure, next to the mean blood pressure. The presence of diastolic hypertension can be interpreted as evidence of a generalized increase in vascular tone [1,7]. The important changes affecting the arterial blood pressures values were showed a long the first 28 days (4 weeks) of the study. In this period of time body suffers a great adaptation to the severe aortic stenosis and reaches a level of hypertension next to the maximum that an organism can tolerate before a fatal vascular accident could happen.
The PRA levels in hypertensive pigs increased 5-fold over normotensive- control levels within 5 days fo the first occlusion of the aorta. Within 30 days of reaching target blood pressure of ≥ 150mmHg of the hypertensive pigs, the PRA levels returned to preocclusion values. Thus, the PRA course in this study was consistent with an aortic coarctation induced model of hypertension in rat [8]. In our opinion, the activity of the renin-angiotensin system could be the main factor in the early development of hypertension. However, other factors must also be involved to explain the chronic and sustained hypertension seen in these animals. In suprarenal aortic constriction, augmented aortic impedance and shear stress might share with the humoral and neural systems a physiopathological role in the increase of arterial pressure [9].
Although elevated mean arterial pressure (MAP) has traditionally been considered to be an important indicator or initiator of cardiovascular risk in hypertension, mounting evidence suggests that increased pulse pressure is as or more important Safar & Boudier [10]. Of particular note, data from surgically created aortic coarctations in animals reveal striking evolutions of wall geometry, structure, and properties that appear to be driven primarily by increased pulse pressure, not MAP [11]. It also appears that the associated arterial adaptations progress at different rates and to different extents both temporally (first at basal rates, then rapidly, then back to nearly normal values) and spatially (from proximal to distal sites) [12]. Although additional experimental data will be needed to understand better these spatio-temporal adaptations, computational fluid–solid-growth models (FSG) [13] offer considerable promise both in the design and interpretation of such experiments and in implicating possible biomechanical mechanisms [14]. Further investigations may allow us to discern which vascular growing factors participate cranially and caudally to the stenosis where flow and pressure are vastly different.
Collateral circulation
The angiographic studies revealed a severe aortic stenosis and an extensive development of collateral circulation affecting the intercostals arteries, intercostals muscles arteries, and internal thoracic. These collateral vessels appeared abnormal and showed evident tortuosity. The angiograms performed showed a clear vascular situation of a total aortic occlusion in some of the animals of the study. Under this situation, the aortic flow had to be diverted through narrower vessels and used the intercostals arteries and intercostals muscle arteries as the first collaterals vessels to be involved. These images also demonstrate the profound influence of aortic impedance and wall shear stress cranial to the occluder. The flow in these collateral vessels was conducted from the highpressure area and high shear stress zone located just before the occluder placement to the low pressure along the thoracic aorta caudal to the occluder. The vascular net is capable of sensing changes within its surroundings, integrating these signals by intercellular communication, and changing itself through the local production of mediators that influence structure as well as function. Thus, the vascular adjustment suffered by the animals of the study and the emergence of molecular and vascular biology in hypertension research has redefined our understanding of the role of the vasculature as a vital organ in the pathogenesis of hypertension [15]. There is no doubt that mechanical conditions to explain development of hypertension are present in our aortic coarctation model although the pathways mediating the response of endothelial cells to hemodynamic stimuli are still unknown [16]. On the other hand, in any adult organism a deficit in the blood flow gives rise to two different mechanisms: angiogenesis and arteriogenesis [17]. In our opinion, these mechanisms are both present in our study based on the observations of the angiogram’s images. These vascular processes are basically different and take place in two distinct types of vessels: capillaries and collateral arteries, respectively. We believe that hypoxia is one of the triggers for the neovascularization from the preexisting vasa vasorum of the aorta, in addition to the inflammatory factors that occur due to the presence of the occluder against the aortic wall. The important role of the vasa vasorum has been described previously where the hypertrophied collateral circulation can be associated to hypertrophied vasa vasorum. This process has been attribuited to the revascularization of an occluded internal carotid artery in atherothrombotic occlusion and in experimental hypercholesterolemia of coronary arteries [18,19]. Arteriogenesis process is defined as the in-situ growth of muscular collateral arteries (arterioles that interconnect two feeding arteries) in response to a physiological stimulus caused by the occlusion of an artery [17,20]. The significant collateral circulation that were present in the animals of our study involved the intercostals arteries and intercostals muscle arteries which showed an increase of lumen and length of these vessels comparing their angiographic images with the control references. There is no doubt that angiogenesis is mainly caused by hypoxia [21]. While this mechanism remains undisputed for angiogenesis, it is most probably not applicable for arteriogenesis because arteriogenesis proceeds in an environment that is not ischemic or is much less ichemic than tissue where the angiogenesis occurs [22,23]. This fact was also reflected in the angiograms where the muscle neoformed arteries were more evident at the cranial area, in front of the occluder.
In conclusion, this study shows that gradual coarctation of the aorta in adults and mature minipigs produces cranial hypertension and an extensive collateral circulation. The collateral vessels, demonstrated by angiography and at necropsy after 8 weeks of the study, can reduce the often-fatal consequences of the large vessel occlusion. Our angiographic studies show vascular processes consistent with arteriogenesis and angiogenesis phenomena bypassing the site of occlusion. We believe that this vascular model based in aortic coartation in adults minipigs can contribute to a better understanding of the vascular changes and angiogenesis and arteriogenesis processes under this clinical condition. Further investigations will contribute to understand the biomechanical mechanisms implicated in the collateral circulation formation.
Acknowledgement
The corresponding author Maria Aguirre-Sanceledonio wish to thank the work and efforts of Dr Theresa W. Fossum, Dr Matthew W. Miller and Dr Jay D Humphrey to support this study as an important base of her doctoral Thesis and thank to the Michael E. DeBakey Institute for Comparative Cardiovascular Sciences and Biomedical Devices, College of Veterinary Medicine, Texas A&M University, College Station, USA to include her as a visitor Researcher in its programme.
References
- Sealy WC, De Maria W, Harris J (1950) Studies of the development and nature of the hypertension in experimental coarctation of the aorta. Surg Gynec Obstet 90: 193-198.
- Guyton AC, Hall JE (1996) Dominant role of the kidneys in long term regulation of arterial blood pressure and in hypertension: the integrated system for pressure control. In: Guyton AC & Hall JE (Eds.), Textbook of Medical Physiology. (9th edn), WB Saunders, Philadelphia, USA, pp. 221-236.
- Karnell J (1968) Coarctation of the aorta. Circulation Supp lV (XXXVII-XXXVIII): V-35-V44.
- Tarkka M, Uhari M, Koskinen M, Heikkinen E (1982) Experimental aortic coarctation in puppies. J Surg Res 33(3): 208-213.
- Warnes CA, Fuster V, McGoon DC (1996) Coarctation of the aorta. (3rd edn), Mayo Clinic Practice of Cardiology, Mosby, Maryland Heights, Missouri, USA, pp.1572-1580.
- Mitchell J, Bohr DF (2005) Experimental hypertension in the pig. In: W de Jong (Ed.), Hypertension Handbook, pp. 148-174.
- Sealy WC (1990) Paradoxical hypertension after repair of coarctation of the aorta: A review of its causes. Ann Thorac Surg 50(2): 323-329.
- Fenandes M, Onesti G, Weder A, Dykyj R, Gould AB, et al. (1976) Experimental model of severe renal hypertension. J Lab Clin Med 87(4): 561-567.
- Salgado HC, Krieger EM (1986) Mechanical and renin-angiotensin system components in acute aortic coarctation hypertension. Hypertension 8: I-133-I-136.
- Safar ME, Boudier HS (2005) Vascular development, pulse pressure, and the mechanisms of hypertension. Hypertension 46(1): 205-209.
- Eberth JF, Popovic N, Gresham VC, Wilson E, Humphrey JD (2010) Time course of carotid artery growth and remodeling in response to altered pulsatility. Am J Physiol Heart Circ Physiol 299(6): H1875-H1883.
- Hayenga HN (2010) Mechanics of atherosclerosis, hypertension-induced growth, and arterial remodeling. Texas A&M University, TX, USA.
- Figueroa C, Baek S, Taylor C, Humphrey J (2009) A computational framework for coupled solid-fluid-growth mechanics in cardiovascular simulations. Comput Methods Appl Mech Eng 198(45-46): 3583-3602.
- Humphrey JD, Taylor CA (2008) Intracranial and abdominal aortic aneurysms: similarities, differences, and need for a new class of computational models. Annu Rev Biomed Eng 10: 221-246.
- Dzau VJ, Gibbons GH, Morishita R, Pratt RE (1994) New perspectives in hypertension research. Potentials of vascular biology. Hypertension 23(6 Pt 2):1132-1140.
- Gibbons GH, Dzau VJ (1994) The emerging concept of vascular remodeling. N England J Med 330 (20):1431-1438.
- Deindl E, Fernandez B, Höfer IE, Van Royen N, Scholz D, et al. (2000) Arteriogenesis, collateral blood vessels, and their development. In: Marcel Dekker (Ed.), Angiogenesis in Health and Disease, pp. 31-44.
- Colon GP, Deveikis JP, Dickinson LD (1999) Revascularization of occluded internal carotid arteries by hypertrophied vasa vasorum: Report of four cases. Neurosurgery 45(3): 634-637.
- Kemény V, Droste DW, Nabavi DG, Schulte-Altedorneburg G, Schuierer G, et al. (1998) Collateralization of an occluded internal carotid artery via a vas vasorum. Stroke 29(2): 521-523.
- Longland CJ (1953) Collateral Circulation of the limb. New Ann Roy Coll Surg Engl 13(3): 161-176.
- Schaper W, Ito WD (1996) Molecular mechanisms of coronary collateral vessel growth. Circ Res 79(5): 911-919.
- Ito WD, Arras M, Scholz D, Winkler B, Htun P, et al. (1997) Angiogenesis but not collateral growth is associated with Ischemia after femoral artery occlusion. Am J Physiol 273(3 Pt 2): H1255-H1265.
- Schaper W, Buschmann I (1999) Arteriogenesis, the good and bad of it. Cardiovasc Res 43(4): 835-837.
© 2020 Maria Aguirre-Sanceledonio. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






