- Submissions

Full Text
Novel Research in Sciences
Chemical Design, Synthesis and Bioefficacy Screening of Insect Growth Inhibitors of Spodoptera Littoralis (Boisd.)
Antar A Abdelhamida1, Elwassimya MM1, Safwat A Arefb2 and Mohamed A Gadb2*
Department of Agriculture, Western Regional Research Center, USA
*Corresponding author:Mohamed A Gadb, Plant Protection Research Institute, Agriculture Research Center, Giza, Egypt
Submission: October 14, 2019;Published: November 20, 2019
.jpg)
Volume2 Issue4November, 2019
Abstract
As a part of our project which aimed to find new growth inhibitors agent’s Spodoptera Littoralis (Boisd.), several inhibitors structurally relevant to the Fenoxycarb as insect growth regulator, and the naturally transpiring juvenile hormone of insects were chemically designed, prepared and evaluated as anti-proliferative agents. This Epihalohydrins derivatives had previously proved to be an extremely active growth inhibitor against Spodoptera Littoralis (Boisd.). Insecticidal bioefficacy data showed that that some compounds are very active against Spodoptera Littoralis (Boisd.) It shows up compounds having naphthalene derivatives 11 and 10 are more active against 2nd instar larvae and 4th instar larvae than another’s of tested compounds.
Keywords: Insecticidal bioefficacy; Spodoptera Littoralis (Boisd.); Insect growth regulator; The toxicity ratio
Introduction
The cotton leafworm Spodoptera Littoralis (Boisd) is consider a polyphagous pest infesting cotton and some important vegetables and field crops in Egypt [1]. Larvae of this insect it has minimum 7-9 generations during the cotton season, additionally infesting more than 29 other crops and vegetables. The achieved control is not successful, when using synthetic insecticides [2] and some biorational agents for example Bacillus thuringiens is Berliner to resist the pest, because of the insect’s high ability to develop resistance toward most of traditional components [3]. Therefore, we need novel compounds that are efficient against this insect, safe to human and ecological well-disposed [4]. The substitutional control techniques that show presage as a potential tactic in S. littoralis the executive’s projects is the utilization of biorational control operators for example synthetic insect growth regulators and those dependent on normally materials [5]. Insect growth regulators are development controllers are ventured to be more secure for useful living beings than regular mixes, and they have been effectively utilized in IPM programs against many tree and little natural product. There is a need for different insecticides having different modes of action Juvenile hormones analogues, sesquiterpenoid moieties arranged and discharged by the corpora allata, are significant insect hormones that standardize a bulky diversity of processes during postembryonic growth and adult reproduction in insects. The main objective of this study was to determinate the toxicity of new growth inhibitors agents against the cotton leafworm, S. littoralis larvae under laboratory conditions.
Result and Discussion
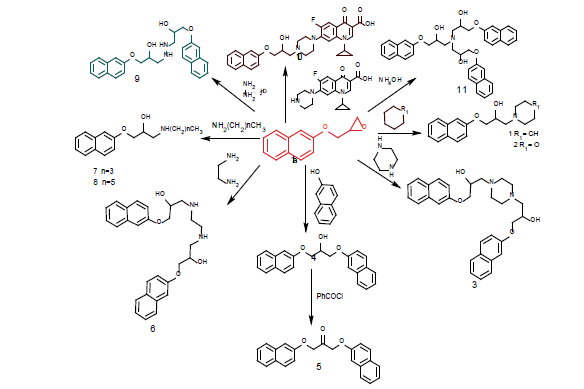
The start reagent can be prepared by the reaction of 2-naphthol with epichorohydrin in presence of catalytic amount of sodium hydroxide afforded of 2-((2naphthyloxy) methyl)- oxiran 1a (Figure 1). The nucleophilic attack of different primary, secondary amines and hydroxyl group to the 2-[(naphthalen-2-yloxy) methyl] oxirane 1a afford of 1-(naphthalen-2- yloxy)-3-(piperidin-1-yl) propan-2-ol
Figure 1:Schematic recurrent selection procedure for obtaining high oil content castor seeds.
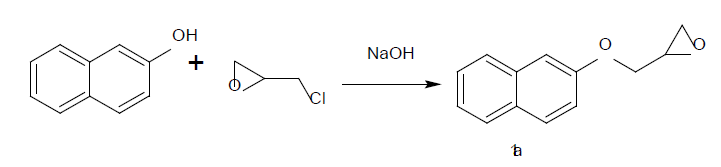
a) 1-(morpholin-4-yl)-3(naphthalen-2-yloxy) propan-2-ol
b) 3,3’-(piperazine-1,4-diyl) bis(1-(naphthalen-2yloxy) propan- 2-ol)
c) 1,3-bis(naphthalen-2-yloxy) propan-2-ol
d) 1,3-bis(naphthalen-2yloxy) propan-2-one
e) 3,3’-(ethane-1,2-diylbis(azanediyl)) bis(1-(naphthalen- 2yloxy) propan-2-ol)
f) 1-(butylamino)-3-(naphthalen-2-yloxy) propan-2-ol
g) 1- (hexylamino)-3-(naphthalen-2-yloxy) propan-2-ol
h) 3,3’-(hydrazine-1,2-diyl) bis (1-(naphthalen-2-yloxy) propan- 2-ol)
i) 1-cyclopropyl-6-fluoro-7-(4-(2-hydroxy-3(naphthalen-2- yloxy) propyl) piperazin-1-yl)-4-oxo-1,4- dihydroquinoline-3- carboxylic acid and
j) 3,3’,3’’-nitrilotris(1-(naphthalen-2-yloxy) propan-2-ol) 11, respectively.
Laboratory Bioassay
The insecticidal bioefficacy of all prepared Epihalohydrins analogues was assessed by the leaf dipping bioassay procedure (Figure 2). Research facility of tested results are accounted here for the used compounds to discover the appropriate concentration that are required to kill half 50% (LC50) of the pests. In this search, five concentrations of each synthesized naphthalene derivatives and 0.1% Triton X-100 as surfactant were utilized. A number total of 10 larvae of Spodoptera Littoralis (Boisd.), almost of the 2nd instar larvae and 4th instar larvae size, Disks (9cm. diameter) of castor bean leaves were dipped in the tested concentrations for 10seconds then left to dry and offered to larvae. Larvae were placed into glass jars (5pounds), every treatment was recreated multiple times (10 larvae per each). Control disks were dunked in distilled water only. In which allowed the larvae to feed on castor bean leaves for 48hr. Then transferred to the untreated ones. Mortality percentages were recorded after 72hr. for all insecticides. Mortality was redressed by Abbott’s formula [6]. The measurements mortality relapse lines were measurably dissected by probit analysis [7]. Harmfulness Index was determined by sun equations [8].
Figure 2:
Insecticidal Bioefficacy Examination
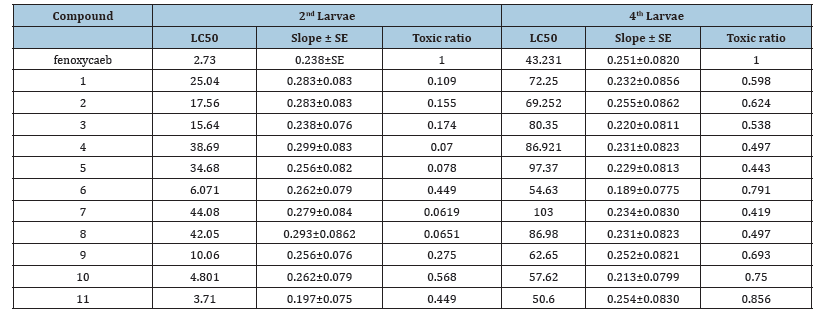
The compounds which prepared have been tested for their insecticidal bioefficacy, demonstrated as follows: compounds from 1-11 were tested against the 2nd instar larvae for their activity as insecticidal, and the outcomes are introduced in Table 1. After 72h of treatment, activity results demonstrated that these compounds show high to low insecticidal activity against the 2nd instar larvae and the LC50 regards stretched out from 3.71 to 44.08ppm, however the LC50 estimation of fenoxycarb was 2.37ppm. This shows that nearly insecticidal bioefficacy of fenoxycarb as insect growth regulators after 72h of test. In which compounds 6, 10 and 11 have generally excellent insecticidal bioefficacy against 2nd because that their LC50 esteems were 6.07, 4.80 and 3.710, respectively, in which the LC50 estimation value of fenoxycarb was 2.37ppm. Varied from strong too weak with LC50 values assorted from 50.60 to 103.01ppm, while the LC50 value of fenoxycarb was 42.231ppm. In which demonstrates that various them have tested compound near that of fenoxycarb as juvenile hormone after 72h of test. For instance, compounds 6, 10 and 11 have generally excellent insecticidal exercises activities against 4th instar larvae because their LC50 values were 54.631, 57.622 and 50.606, respectively, and the LC50 value of fenoxycarb was 43.231ppm.
Table 1:Compounds from 1-11 were tested against the 2nd instar larvae for their activity as insecticidal, and the outcomes are introduced.
Structure Framework Relationship
It shows up from the general search structure of the prepared naphthalene derivatives compounds of 11and 10 are more active against 2nd instar larvae and 4th instar larvae than another’s of tested compounds. It additionally demonstrated that exacerbates that contain an exceptionally ether group in the structure claim a high insecticidal bioefficacy. Thus, compounds 6, 9, 10, and 11 more active than compounds 1, 2, 3, 4, 7 and 8 in insecticidal bioefficacy.
Acknowledgement
This work was financially supported by the plant protection institute, agriculture research center of Egypt, and chemistry department, Sohag University.
References
- Marwa FK, Ali AM (2017) Impact of some essential plant oils and insect growth regulators on immature stages of Spodoptera Littoralis (Boisd.) (Lepidoptera: Noctuidae) in Egypt. J Plant Prot and Path 8(11): 561-570.
- Amira SMI (2019) Sterilizing activity of the insect growth regulator, lufenuron on Drosohpila melanogaster (Meigen). J Plant Prot and Path 10(5): 297-302.
- Gelbic I, Adel MM, Hussein HM (2011) Effects of nonsteroidal ecdysone agonist RH-5992 and chitin biosynthesis inhibitor lufenuron on Spodoptera Littoralis (Boisduval, 1833). Central European Journal of Biology 6: 861-869.
- Nasr HM, Badawy ME, Rabea EI (2010) Toxicity and biochemical study of two insect growth regulators, buprofezin and pyriproxyfen, on cotton leafworm Spodoptera Littoralis. Pestic Biochem Physiol 98(2): 198-205.
- Wheeler DE, Nijhout HF (2003) A perspective for understanding the modes of juvenile hormone action as a lipid signaling system. Bioessays 25(10): 994-1001.
- Abbott WS (1925) Method of computing the effectiveness of an insecticide. Journal of Economic Entomology 18(2): 265-267.
- Finney DJ (1952) Probit analysis: A statistical treatment of the sigmoid response curve. Annals of the Entomological Society of America 45(4): 686.
- Sun YP (1950) Toxicity index-an improved method of comparing the relative toxicity of insecticides. J Econ Entomol 43(1): 45-53.
© 2019 Mohamed A Gadb. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






