- Submissions

Full Text
Novel Research in Sciences
Chemical Design and Effects of New Insect Growth Regulators as Potential Insecticidal Agents on Spodoptera littoralis (Boisd.)
Mohamed A Gad1, Elwassimy MM2, Safwat A Aref1 and Antar A Abdelhamid2
1Research Institute of plant protection, Agriculture Research Center, Egypt
2Department of chemistry, Sohag university, Egypt
*Corresponding author:Mohamed A Gad, Research Institute of plant protection, Agriculture Research Center, 12112 Giza, Egypt
Submission: November 05, 2019;Published: November 12, 2019
.jpg)
Volume2 Issue3November, 2019
Abstract
Comparative studies of the effects of seven compounds and fenoxycarb (ecdysone agonist, an insect growth regulator), were conducted on Spodoptera littoralis (Boisduval, 1833). The compounds, orally administered, caused larval mortality proportional to the concentrations in the food source. Synthesized compounds initiated precocious molting and reproduction. In both cases, larvae were unable to complete the molting process and died in the old larval cuticle. Larvae contaminated by sublethal doses completed their development to adulthood. fenoxycarb is more active than is synthesized compound. LC50 for fenoxycarb is 25.943 ppm and for compound 2 is 26.505ppm. Application of fenoxycarb in the second half of embryogenesis caused precocious molting of eclosed larvae of the 2nd instar. Some morphological changes in the process of larval–pupal transformation was also observed
Keywords: Spodoptera littoralis (Boisd.); Fenoxycarb; Toxicity; Insect growth regulator
Introduction
The Egyptian cotton leafworm Spodoptera littoralis (Boisduval, 1833) is a large, polyphagous insect known to destroy more than 100 different species of plants. For this reason, there is strong interest to find new methods for its control. New hormone-based compounds, including nonsteroidal analogues of ecdysone and insect growth regulators like lufenuron, appear to be useful. The nonsteroidal ecdysone agonist dibenzoyl hydrazine (RH- 5992 or fenoxycarb) has been tested on different larval stages for a number of lepidopteran species [1]. RH-5992 has been shown to induce precocious molting in all tested lepidopteran larvae, including the tobacco hornworm, Manduca sexta, and the spruce budworm, Choristoneura fumiferana [2]. Although this nonsteroidal ecdysteroid agonist was developed with the aim of disturbing larval development, substantial effects have been observed on lepidopteran reproduction. Studies carried out on several species belonging to various families (Crambidae, Noctuidae, Pyralidae, and Tortricidae) have shown that tebufenozide affected ovarian development [3-5] and reduced fecundity (total of eggs laid) [6] and the fertility of both females [7] and males [8]. Direct and latent effects of two chitin synthesis inhibitors, lufenuron and lefenuron/deltanet, on S. littoralis larvae were studied [9]. Lufenuron proved to be more toxic than lufenuron/deltaned. Both compounds produced delayed effects on surviving treated larvae, and some larvae failed to pupate successfully. In some cases, the delayed effects were manifested in the pupal or adult stages, expressed by death at either stage or significant reduction in reproductive potential of moths resulting from treated larvae [10].
Materials and Methods
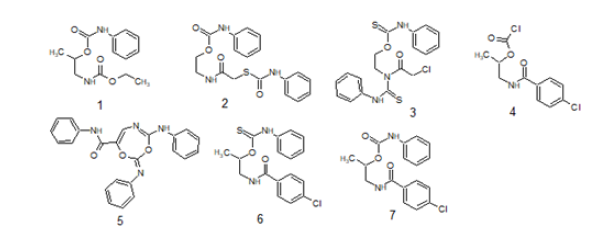
Estimating of the MP. for all prepared target compounds was completed on a Fisher-John mechanical technique. By utilizing a Vario EL C, H, N, S analyzer, basic examinations (C, H, N, and S) were elucidated. On a Pye-Unicam SP3-100 spectrophotometer IR spectra were gotten by utilizing the KBr disc technique. 1H NMR and 13C NMR spectra were estimated on Bruker 400MHz spectrometers utilizing tetramethylsilane (TMS) as a source of perspective and concoction movements were accounted for as ppm. By utilizing a Jeol JMS-400 mass spectrum were completed. Fenoxycarb juvenile hormone analogues as an insect growth regulators insecticide was buy from Sigma-Aldrich. The numbers of S. littoralis insects were gathered from cotton leave worm, fields of Assiut University. Toxic activity of the seven compounds comparing with fenoxycarb as reported insecticide was tested against the instar larvae of S. littoralis. namely by 1-[(ethoxycarbonyl)amino] propan-2-yl phenylcarbamate (1), 2-({[(phenylcarbamoyl)sulfanyl] acetyl}amino)ethyl phenylcarbamate (2), O-{2-[(chloroacetyl) (phenylcarbamothioyl)amino]ethyl} phenylcarbamothioate (3), 1-{[(4-chlorophenyl)carbonyl]amino}propan-2-yl carbonochloridate (4), (2E)-N-phenyl-4-(phenylamino)-2- (phenylimino)-1,3,5-dioxazepine-7-carboxamide (5), O-(1-{[(4- chlorophenyl)carbonyl]amino}propan-2-yl) phenylcarbamothioate (6) and 1-{[(4-chlorophenyl)carbonyl]amino}propan-2-yl phenylcarbamate (7) (Figure 1).
Figure 1:Purposed compounds that tested against S. littoralis.
Laboratory bioassay
The method that measure toxicity of the target compounds was tested by leaf dipping bioassay. Results of research facility screening to discover the suitable concentrations of the objective target compounds which are deformation in the insect to kill half 50% LC50 of instar larvae were proclaimed here. Five concentrations of arrangement of each synthesized compound in addition to 0.1% Triton X-100 as a surfactant were used. The number of ten 2nd instar larvae and 4th instar larvae of insects, nearly have the same size, plates (9cm. distance across) of castor bean leaves in which dunked in the objective treatment concentrations for 10 seconds then left to dry and offered to larvae, which starved for 4-6 hours before treatment). Larvae were put into glass containers (5 pounds), every treatment was reproduced multiple times (10 larvae for each). Control was dunked in distilled water only. The larvae were permitted to benefit from treated plates for 48hr., then transferred to the untreated ones. Mortality percentages were recorded after 72hr. for all insecticides. Mortality was redressed by Abbott’s formula [11]. The doses mortality relapse lines were statistically investigated by probit analysis [12]. Toxicity Index and Relative Potency determined by Sun equations
Slope esteems and middle deadly focused concentrations LC50 of the title target compounds were determined through a Probit relapse investigation program and recorded in (ppm). Were inundated for 10 seconds in each concentration multiple times (3 times). Pests which treated were leaved to dry at room temperature for about half hour. Control clumps of utilized pests were likewise used. The insecticidal action trial of each compound was rehashed multiple times (2 time) and the gotten data were rectified by Abbott’s equation [11]. By utilizing a modernized probit relapse investigation program, middle deadly fixations (LC50) and incline estimations of objective target compounds were figured and revealed as (ppm) [12].
Result and Discussion
Experimental
A Fisher-Johns instruments were practiced registering the melting points of every prepared compounds. Infra-red and elemental analyses (C, H, N, and S) were achieved through a PyeUnicam SP3-100 spectro-photometer utilizing the KBr disk strategy and a Vario EL C, H, N, S analyzer, separately. A Bruker 400 MHz spectrometer was utilized to measure DEPT 135 spectra and the 1H and 13C NMR spectra within the TMS as an interior standard. Reaction headway and perfection of the prepared sections were checked by thin layer chromatography.
Toxicological activity test for the 2nd instar larvae
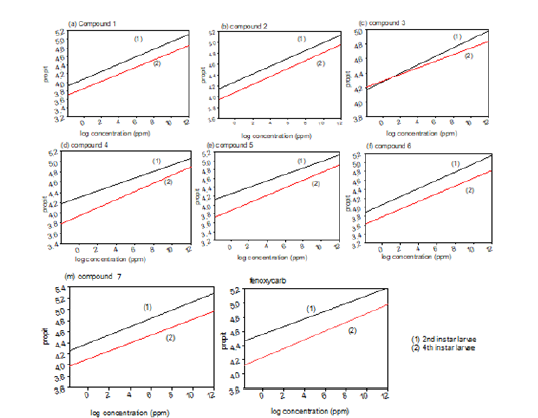
As shown in Table 1 target compounds were tested of their activity as insecticides in which shown beneath. Seven previously mentioned compounds displayed strong to weak toxic action against the 2nd instar larvae in light of the fact that several of them were closed in activity than fenoxycarb after 72hr. of the test with LC50 qualities differ from 26.505 to 43.414ppm for 2nd instar larvae for example, LC50 values of compounds 1, 2, 3, 4, 5, 6, and 7 were 36.546, 26.505, 27.622, 43.414, 39.720, 30.478, and 33.424ppm, respectively in a specific order, and LC50 of fenoxycarb was 25.943 ppm. From outcomes in over, the toxicity of compound 2 against the S. littoralis larvae 26.505ppm after 72 hours of the test on the grounds was closed than estimation of reported fenoxycarb (Figures 2 & 3).
Figure 2:Morphological malformations of Spodoptera littoralis (Boisduval, 1833) caused by tested compounds. A. Morphological changes caused by tebufenozide (pupae with larval head, larval legs and shortened malformed wings; B. Morphological changes caused by lufenuron (pupae with larval head, larval legs, nondeveloped wings and remnants of larval prolegs); C. Normal pupae (control).

Figure 3:Compounds 1-7 and reference juvenile hormone analogue fenoxycarb as insecticidal activities against the 2nd and 4th instar larvae of S. littoralis after 72h of treatment.
Toxicological activity test for the 4th instar larvae
As shown in Table 1 target compounds were tested for their activity as insecticides and this is shown beneath. Thirteen previously mentioned compounds displayed strong to weak toxic action against the 4th instar larvae in light of the fact that various them were active than fenoxycarb after 72hrs of the treatment in which LC50 values changed from 66.253 to 120.25ppm while LC50 values of fenoxycarb is 59.914ppm. Compounds 1, 2, 3, 4, 5, 6 and 7 gave a high toxicity with LC50 values of 102.322, 67.908, 66.253, 120.25, 91.406, 97.360 and 86.203ppm. Comparing with fenoxycarb compound 3 shown that the closed in toxicity while compounds 2, 5 and 6 give a very good activity, respectively.
Structure-activity relationship
As a resumption of our search, the structure-activity relationships were accounted for here as indicated by the poisonous activity peaks in Table 1 underneath and Figure 2 too. It is demonstrated that the 4-aminophenol derivatives 2 and 3 is progressively active against of S. littoralis than different compounds that prepared. The large activity related with compounds 2 might be because of the closeness of the carbamate and sulfer group moiety independently in their chemically structure and the general qualities of the synthesized compounds.
Table 1:Insecticidal activity of compounds 1-7 and juvenile hormone analogue fenoxycarb against the larvae of spodoptera littoralis (Boisd.), after 72h of treatments.

Acknowledgement
This work was financially supported by the plant protection institute, agriculture research center of Egypt, and chemistry department, Sohag University.
References
- Smagghe G, Salem H, Tirry L, Degheele D (1996) Action of a novel insect growth regulator tebufenozide against different developmental stages of four stored product insects. Parasitica 52(2): 61-69.
- Dhadialla TS, Carlson GR, Le DP (1998) New insecticides with ecdysteroidal and juvenile hormone activity. Ann Rev Entomol 43: 545-569.
- Carlson GR, Shadialla TS, Hunter R, Jansson RK, Jany CS, et al. (2001) The chemical and biological properties of methoxyfenozide, a new insecticidal ecdysteroid agonist. Pest Manag Sci 57(2): 115-119.
- Hami M, Taibi F, Smagghe G, Soltani Mazouni N (2005) Comparative toxicity of three ecdysone agonist insecticides against the Mediterranean flour moth. Commun Agric Appl Biol Sci 70(4): 767-773.
- Retnakaran A, Hiruma K, Palli SR, Riddiford LM (1995) Molecular analysis of the mode of action of RH-5992, a lepidopteran-specific, non-steroidal ecdysteroid agonist. Insect Biochem Mol Biol 25(1): 109-117.
- Retnakaran A, Gelbic I, Sundaram M, Tomkins W, Ladd T, et al. (2011) Mode of action of the ecdysone agonist tebufenozide (RH-5992), and an exclusion mechanism to explain resistance to it. Pest Manag Sci 57(10): 951-957.
- Palli SR, Retnakaran A (1999) Molecular and biochemical aspects of chitin synthesis inhibition. In: Jollès P & Muzzarelli RAA, (Eds.), Chitin and Chitinases, Birkhaüser Verlag, Basel, Switzerland, pp. 85-98.
- Palli SR, Primavera M, Tomkins W, Lambert D, Retnakaran A (1995) Age-specific effects of a nonsteroidal ecdysteroid agonist, RH-5992, on the spruce budworm, Choristoneura fumiferana (Lepidoptera: Totricidae). Eur J Entomol 92: 325-332.
- Smagghe G, Degheele D (1994) Action of a novel nonsteroidal ecdysteroid mimic, tebufenozide (RH-5992), on insects of different orders. Pestic Sci 42(2): 85-92.
- Smagghe G, Degheele D (1994) The significance of pharmaco kinetics and metabolism to the biological activity of RH-5992 (tebufenozide) in Spodoptera exempta, Spodoptera exigua, and Leptinotarsa decemlineata. Pest Biochem Physiol 49(3): 224-234.
- Abbott WS (1925) Method of computing the effectiveness of an insecticide. J Econ Entomol 18(2): 265-267.
- Finney DJ (1925) Probit analysis: a statistical treatment of the sigmoid response curve. Cambridge University Press, Cambridge, UK.
© 2019 Mohamed A Gad. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






