- Submissions

Full Text
Novel Research in Sciences
Bactericidal Cellulosic Textile Materials with Silver Nanoparticles
Yunusov EK1*, Sarymsakov AA1, Mulajanova SV2, Nabieva IA2 and Rashidova SS1
1Department of Polymer Chemistry and Physics, Uzbekistan
2Tashkent Institute of Textile and Light Industry Tashkent, Uzbekistan
*Corresponding author:Yunusov EK, Institute of Polymer Chemistry and Physics, Uzbekistan
Submission: May 22, 2019; Published: July 11, 2019
.jpg)
ISSN : 2688-836XVolume1 Issue3
Abstract
Stabilized silver nanoparticles, in solutions sodium-carboxymethyl cellulose (Na-CMC), were synthesized and their structure and physico-chemical properties were studied. The forms and sizes of silver nanoparticles were studied by atomic force microscopy and transmission electron microscopy methods, which silver nanoparticles are loaded in solutions CMC and grafted cotton fabrics. Investigations show that spherical silver nanoparticles with size 5-35nm and with content of 0.0085% mass. in cotton fabrics has a high bactericidal activity. Stabilization of silver nanoparticles helps to save the bactericidal and bacteriostatic activity during washing the cotton fabrics and textiles on their basis.
Keywords: Biomaterials; Silver nanoparticles; Sodium-carboxymethylcellulose; Cellulose fibers; Cotton fabrics
Introduction
Presently, there is great practical interest in the elaboration of cellulose fibers and textile biomaterials and products on their bases possessing a high bactericidal activity against pathogenic fungus and bacteria [1]. Bactericidal and bacteriostatic activity in the bases of cellulosic biomaterials and their products may take place by incorporating silver ions in their structure and following their reduction into nanoparticles, their interaction with the polymer matrix is carried out through the formation of co-ordinational bonds [2]. Currently, it is determined that silver and its compounds can inhibit the growth of and kill more than 650 types of bacteria, viruses, and fungi, consequently keeping up with the microelement, which is an essential part of the fabric of all living organisms [3]. Silver nanoparticles have an extremely large specific surface area, which increases when in contact with bacteria, viruses, and fungi. This significantly increases their bactericidal activity by decreasing the sizes of silver nanoparticles and by increasing their surface area to volume ratio [3,4]. The aim of this investigation is to elaborate on the presence of silver nanoparticles in bactericidal, antifungal fibers, and textile biomaterials and products, as well as to study their structure and properties.
Experimental Section
Cellulosic fibers, cotton fabrics (c/f), textile fabric and purified Na-carboxymethyl celluloses (Na-CMC) with degree of substitutions (DS)=0.65-0.85 and degree of polymerizations (DP)=200-600 were used as a polymer matrix [5,6]. An aqueous solution of silver nitrate (AgNO3) was used for the formation of silver nanoparticles in solutions Na-CMC. As strain pathogen microorganisms of humans and animals were used Staphylococcus epidermidis, Candida albicans.
Experimental set up
For the formation stabilized silver nanoparticles 0.1-0.4% aqueous solutions of purified samples of Na-CMC with various DS and DP was used after removal the gel fraction by centrifugation on laboratory centrifuge at 4000rev/min for 10 minutes. For this purpose, calculated quantity of 0.1-0.001mol/l aqueous solutions of AgNO3 was added drop by drop to Na-CMC solution liberated from gel fraction with intensive stirring, until obtain homogeneous of solution Ag+CMC-. Photochemical reduction of silver ions in the structure of Ag+CMC- until nanoparticles was performed at 25 ˚C, by irradiating high pressure mercury lamp DRSH-250.
In order to obtain dispersed silver nanoparticles UZDN-1, Y-4, 2 type dispersers were used. By means of work up treating cellulosic fibers, cotton fabrics and textile products with solutions contained the stabilized silver nanoparticles and after their subsequent heat treatment obtained crosslinked water-insoluble compositions of cellulosic fibers and cotton fabrics with antibacterial and antifungal properties.
The size and shape of silver nanoparticles in the Na-CMC solution was controlled by spectrophotometer method in UVspectroscopy, spectrophotometer model Secord M210 in the wavelength range from 200 to 900nm. The size, shape and size distribution of the silver nanoparticles in the cotton fabrics were determined by electron microscopy, type transmission electron microscope TEM-100 (Ukraine) and atomic-force microscope AFM- 5500 (Austria). The variation coefficients were determined by the calculation corresponding micrographs on Math Cad program. Content of silver nanoparticles in cotton fabric were determined by atomic absorption spectroscopy model of spectrophotometer PERKIN-ELMER 3030 B (USA) with a flame analyzer (acetyleneair).
Bactericidal activity of cotton fabrics contained silver ions and nanoparticles were studied by microbiological method [7] at the pathogenic bacterium Staphylococcus epidermidis and fungus Candida albicans.
Result & Discussion
Results
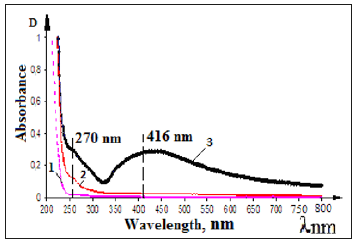
At the first step of investigation solutions of Na-CMC with different DS and DP were obtained to form the stabilized silver nanoparticles. The formation of silver nanoparticles in the structure of samples Na-CMC were carried out at 0.2-0.4% concentrate solutions to provide them free access to both interfere and intermolecular spaces of cellulose textiles after their impregnation. Formation of nanoparticles from silver ions in the structure of dilute solutions of Na-CMC with different DS-(0.65-0.85) and DP-(200-600) were carried out by photochemical reduction of silver ions [8,9]. It has been established that adding silver ions in Na-CMC solutions causes to increase the viscosity of system due to decreasing the solubility of Ag+CMC- and appearance of coordination bonds between molecules with formation of poly complexes. Increasing the relative viscosity of Na-CMC contained silver cations dependence on decreasing the solubility due to formation of intermolecular coordination bonds between the silver ions and carboxylate anions in macromolecules of Na-CMC [10]. Synthesized silver nanoparticles, by photochemical method, in Na-CMC solution provided high stability and didn’t come to agglomeration during keeping at long times, to compare with nanoparticles reduced from silver ions in aqueous solutions by chemical agents. After UV-irradiation solutions of Na-CMC contained silver ions formed enough stable colloidal systems of Nano silver pale- yellow color, contained at the optical spectra maximum at λmax=416nm, which characterizes nanoparticles of silver with sizes 5-25nm [11]; (Figure 1), curve -3. It can be seen in the spectra of initial solutions of Na-CMC and Ag+CMC- at the 250-900nm optical region was no changes observed (Figure 1), curves-1,2. With increasing the time of photolysis the color of solutions is changed from pale yellow to brown. According to literature such changes are probably dependent on with an increasing the number and size of formed silver nanoparticles.
Figure 1:UV-VIS absorption spectra of the samples 1) Na-CMC 2) Ag+CMC- 3) Ag0CMC Time of UV-irradiation for samples 25min. [Na-CMC] =0.2% [AgNO3]=1•10-2 mol/l.
For the confirm, this assumption absorption spectra were taken at the different times of irradiation of systems Ag+CMC- at concentration of Na-CMC- 0.2% and 1.10-2mol/l silver nitrate. (Figure 2) shows the UV-spectrum solutions of Na-CMC contained silver nanoparticles which obtained at different times of photo irradiations. One can see in Figure 2. After 5-minute photo irradiation in the spectrum observed the shoulder at region λmax=270nm, which could be attributed to stable polyanions charged silver clusters, approximately Ag82+ [12,13]; (Figure 2), curve-2. After 15,20 minutes photolysis at the spectrum observed increase the intensity of the absorption band at λmax=270nm, which depends on with the formation of large stabilized clusters of silver with sizes 2-8nm [13]; (Figure 2), curve - 2,3.
Figure 2:The absorption spectra of photochemical reduced silver ions samples Ag+CMC-. Concentration of [Na-CMC] =0.2 %; and [AgNO3] =1•10-2mol/l. Time of UVirradiation 0(1) 5(2) 15(3) 20(4) 30(5).
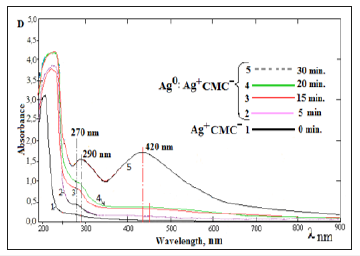
Further irradiation brings to the appearance of new absorption peaks with maximums at regions λmax=290nm and λmax=420nm which belongs to larger clusters and nanoparticles of silver with sizes 5-35nm [11]. Homogeneity formed nanoparticles in different sizes can be obtained because of the macromolecules of Na- CMC covers the silver nanoparticles create charged shell around them to prevent nanoparticles’ aggregation. Commonly, when polymer concentration is high, it tends to stabilize nanoparticles. Increasing the local concentration of Na-CMC on the surfaces of silver nanoparticles created on the one hand, supports electrostatic and steric stabilization, and on the other hand-creates a condition in which don’t completely exclude interaction generated radicals by UV-photolysis of Na-CMC. To confirm the stability of formed silver nanoparticles in polymer matrix the UV-spectra of Na-CMC solutions containing stabilized silver nanoparticles has been taken which (Figure 3), keeping for the different periods of time.
Figure 3:UV-absorption spectra of solutions of Na- CMC, contained silver nanoparticles for keeping during sometimes. 1) 1-month. 2) 2-months. 3) 6-months.
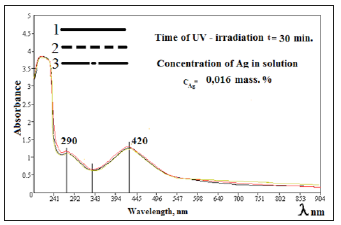
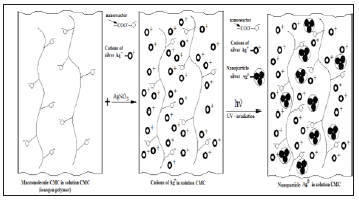
One can see from Figure 3, form and content of silver nanoparticles in Na-CMC solutions, at during keeping times at home conditions absorption peak practically unchanged. Based on the experimental results it could be concluded that, with depending on the molecular weight, degree of substitution and the ratio components of Na-CMC and AgNO3 able to control the size and shape of silver nanoparticles which will forms in solutions of Na-CMC by photochemical reduction [14]. Analyzing spectroscopic data nanocomposites based on silver and Na-CMC, were suggested that the negative ions of carboxymethyl group, is “catcher” for the positive charged of silver ions. Well known that on basis of the photographic process, halogens of silver are formation of colloidal silver particles with prolonged irradiation of photons above the band gap. This electron-stimulated process of formation of colloidal particles had been explained on the basis of Mott-Gurney theory [15], the essence which as follows. During the photochemical reduction the optically generated electron migrates and cached in electron catcher at the interfaces and near the surface (Figure 4). Cations of silver attracts to carboxymethyl anion of CMC to form an ionic bond. Reduction reaction begins with connected silver cations-at “nanoreactors” with formation clusters, which turn into nanoparticles, due to recovery of cations near nanoreactors.
Figure 4:Assumption scheme of formation nanoparticles of silver in structure Na-CMC.
This is the first step in the sequence which the electrons and interstitial atoms catching, forming clusters and silver nanoparticles, which make on basis latent image. For the purpose to determine the form and size of silver nanoparticles in the structure of Na- CMC, carry out investigation’s samples by atom-force microscope. Received data are presented in Figure 5. You can see from AFM images, that at low concentration of silver ions formed spherical polydisperse nanoparticles with sizes 2-8nm. With increasing ions of silver concentrations after photolysis forms monodisperse nanoparticles of silver with sizes 5-35nm. This can be explained by the fact that at the same time silver cations in compositions’ CMC connected and disconnected with carboxylic anions (nanoreactor) [15] of CMC are reduced by increasing the time of photolysis.
Figure 5:AFM microphotographs of solutions Na-CMC, contained silver nanoparticles (a), (b) and size distribution of nanoparticles (c), (d). Concentration of [Na-CMC] =0.2 %; [AgNO3] =1•10-2mol/l. Time of UV-irradiation 20 (a) and 30min (b).
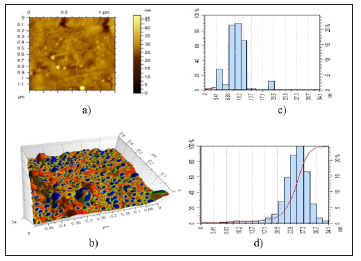
These processes occur with different speed, which probably bring to an increase in the polydispersity of formed nanoparticles. Thus, the size and shape of silver nanoparticles formed by the photochemical reduction of silver cations, at systems Ag+CMC-, depend on DS and the concentration of solution of Na-CMC, and concentration of Ag+ and times of photochemical irradiation. Also was found that by keeping the concentration of carboxylate anions in the solution of Na-CMC with increasing content of silver cations in system by the same time occur increase the size of silver nanoparticles and their contents. There is also observed changing the form of silver nanoparticles formed from spherical to rod like structure [13]. Based on the results of studies selected next conditions for the formation of homogeneous in size of silver nanoparticles. Time of UV-irradiation 30 minutes, the contents of Na-CMC in solution 0.2%, content of AgNO3 in solution 0.016wt. %. In selected conditions forms spherical stable silver nanoparticles with sizes 5-35nm.
At the present time, the joint efforts of chemists, biologists and physicians are attention on the problem of giving polymeric materials additional therapeutic and prophylactic properties by forming the silver nanoparticles into structure of drugs [6]. We have obtained biodegradable hydrogels, films and cotton fabrics and productions with a high bactericidal activity on the base of Na-CMC and cellulose contained silver nanoparticles [16]. For the aim of obtaining the bactericidal cotton fibbers, materials and fabrics they have been grafted with Ag0CMC solutions. Therefore, at grafting low concentration of Na-CMC solution and the sizes of nanoparticles and ions of silver they able to inter inter fiber and intermolecular free spaces of materials and fabrics obtained from cellulose.
Obtained wet cotton fabrics and products subjected to additional UV-irradiation, where occurred restoration of the unreacted silver ions into structure of Na-CMC matrixes [17-20]. We have investigated the samples with transmission electron microscope. The result data are shown in Figure 6. You can see from Figure 5, the size of spherical silver nanoparticles is at range 2-30nm, their content in cotton fabrics was 0.0086wt.%. Supposed scheme of formation of silver nanoparticles in the structure of cotton fabric, complex fibers and elemental of cellulose fiber are shown in Figure 7. When cotton fabrics, complex of fibers and elemental fibers of cellulose (Figure 7) grafts with the mixture solutions of Ag0CMC and CMC-Ag+ they inter between free spaces of fibers
1. And between free spaces of complexes of fibers including in the structure of fabric
2. It was found that the macromolecule of Na-CMC, contained nanoparticles and ions of silver able to inter between free spaces fibrils elemental of fiber
3. Cellulose, which are a “trap” for the nanoparticles and ions of silver.
Figure 6:TEM images of initial cotton fiber (a) and silver nanoparticles on the surface cotton fiber (b).
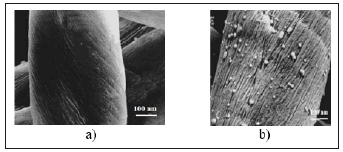
Figure 7:TEM images of initial cotton fiber (a) and silver nanoparticles on the surface cotton fiber (b).

After repeated photoirradiation of wet fiber the silver ions which situated on between strings, between fibers and between fibrils space reduction until nanoparticles in the structure of Na-CMC which, after drying changes into an insoluble state by formation of intermolecular hydrogen and covalent bonds between the carboxyl and hydroxyl groups of the macromolecules of Na-CMC and cellulose. This explained by the silver nanoparticles stability in the structure of fibers and fabrics based on with repeated washings [21].
Conclusion
It was found that the grafted cotton fabrics and products based on with stabilized silver nanoparticles contribute to provide bactericidal and bacteriostatic properties. Bactericidal activity of fabrics depends on the content, size and form of the silver nanoparticles in the structure of fabrics. Stabilization of silver nanoparticles in the structure of the polymeric matrix brings to keep their bactericidal and bacteriostatic activity during washing up the cotton fabrics and products. Based on the results of investigation it was found the optimum conditions for obtain bactericidal and bacteriostatic cotton fabrics and textiles.
Acknowledgment
The work was performed as part of the innovation project No. IZ-20170920233 “Development of technology and mastering the production of nanostructured, bio-soluble, antiviral eye medicinal films “GlazAvir”” Ministry of Innovative Development of the Republic of Uzbekistan.
References
- Cusina MI, Kostyuchenok BM (1990) Wounds and wound infection: Textbook for physicians. In: 2nd edn. M.: Medicine, p. 529.
- Lee HJ, Yeo SY, Jeong SH (2003) Antibacterial effect of nanosized silver colloidal solution on textile fabrics. J Mater Sci 38(10): 2199-2204.
- Oleg M Physiological effect of silver nanoparticles on the human body. www. nanonewsnet. ru. (General Web sites)
- Blagitko EM, Burmistrov VA, Kolesnikov AP, Mikhailov YU, Rodionov PP (2004) Silver in medicine. Novosibirsk Science Center, Russia, p. 256.
- Sherbakov AB (2006) Preparations of silver yesterday, today and tomorrow. Pharmaceutical journal 5: 45-57.
- Mirzoeva AE (2002) Methods for general bacteriology. Moscow, p. 62.
- Oltarjevskaya ND, Ryltsev VV (1991) New ways of getting treatment textile materials. Moscow, p. 93
- Yunusov E, Rashidova SS, Sarymsakov AA (2009) Synthesis and physicochemical properties of silver nanoparticles stabilized by solution of Nacarboxymethylcellulose. Uzbek Chemical Journal 3: 15-20.
- Sergeev BM, Kyruhin MV, Bachov FN, Sergeev VG (2001) Photochemical synthesis of silver nanoparticles in aqueous solutions of polycarboxylic acids. Effect of polymer matrix to the size and shape of the particles. Vestn Mosk Univ Ser 2 Chemistry 42. 5: 308-314.
- Piatnitskiy IV, Suchan VV (1975) Analytical chemistry of the silver. Moscow, p. 256.
- Lopatina LI, Sergeev VG (2010) Effect of molecular weight and structure of polycyclic acid on the formation of “blue silver”. Vestnik Moscow University Series 2 Chemistry 5: 398-401.
- Belloni J, Mostafavi M, Remita H, Marignier JL, Delcourt MO (1998) Radiation-induced synthesis of mono- and multi-metallic clusters and nanocolloids. New J Chem 22: 1239.
- Kreibig U, Vollmer M (1995) Optical properties of metal clusters. Springer, Berlin, p. 533.
- Oksigendler BL, Turaeva NN, Yunusov E, Sarymsakov AA, Rashidova SS (2009) The mechanism of the influence of ultraviolet radiation on the growing and properties of silver nanoparticles in the polymer solutions. Tashkent, Uzbekistan, pp. 82-85.
- Yunusov E, Atakhanov AA, Ashurov NA, Sarymsakov AA, Rashidova SS (2011) Physico-chemical studies of cotton cellulose derivatives, containing silver nanoparticles. Journal Chemistry of Natural Compounds 3: 370-373.
- Sarymsakov AA, Yunusov KE, Atakhanov AA, Rashidova SS (2011) Biomedical polymers and perspectives of their creation. Pharmaceutical Journal 3: 29-37.
- Feng QL, Wu J, Chen GQ, Cui FZ, Kim TN, et al. (2000) A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J Biomed Mater Res 52(4): 662-668.
- Dubas ST, Kumlangdudsana P, Potiyaraj P (2006) Layer-by-layer deposition of antimicrobial silver nanoparticles on textile fibers. Colloids Surf A: Physicochem Eng Aspect 289(1-3): 105-109.
- Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, et al. (2005) The bactericidal effect of silver nanoparticles. Nanotechnology 16(10): 2346-2353.
- Yunusov KE, Atakhanov AA, Sarymsakov AA, Rashidova SS, Lobanova KV, et al. (2010) Silver nanoparticles were formed on the polymer matrixes and their bactericidal properties. Pharmaceutical Journal 1: 55-59.
- Lee HJ, Jeong SH (2005) Bacteriostasis and skin innoxiousness of nanosized silver colloids on textile fabric. Textile Research Journal 75(7): 551-556.
© 2019 Yunusov EK. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






