- Submissions

Full Text
Novel Approaches in Cancer Study
Twirl and Whirl-Myopericytoma
Anubha Bajaj*
Department of Pathology, Panjab University, India
*Corresponding author:Anubha Bajaj, Department of Pathology, Panjab University, India
Submission: April 30, 2024;Published: May 10, 2024

ISSN:2637-773XVolume7 Issue5
Opinion
Myopericytoma emerges as a distinct myoid neoplasm delineating perivascular distribution of tumour cells. The neoplasm configures a morphological continuum with myofibroma. Myopericytoma is comprised of bland, spindle shaped myoid cells configuring a concentric pattern circumscribing innumerable, miniature vascular articulations. Myofibroma expounds a biphasic tumour pattern and exhibits primitive cellular zones admixed with frequent mitotic figures, tumour necrosis and focal calcification. Aforesaid cellular elements are encompassed within hyalinised nodules constituted of myoid spindle shaped cells. Myopericytoma and myofibroma expound chromosomal mutations within PDGFRB gene. Cellular myofibroma or myopericytoma exemplifies SRF-RELA genetic fusion. Nomenclature of infantile hemangiopericytoma is not recommended. Myopericytoma commonly arises within adult subjects although no age of disease emergence is exempt. Myofibroma may emerge as a congenital lesion or occur within 2 years or within adults and expounds a male predilection [1,2]. Myofibromatosis preponderantly implicates infants and young children wherein majority (~88%) of neoplasms occur <2 years. Solitary infantile myofibromatosis is commonly encountered within male subjects whereas multi-centric infantile myobromatosis expounds a female predilection [1,2]. Myopericytoma and myofibroma preponderantly emerge as cutaneous lesions or may be confined to subcutaneous tissues of extremities, trunk or head and neck region. Exceptionally, neoplasm is encountered within diverse viscera or intracranial sites. Infants with myofibromatosis delineate lesions within various visceral locations as hepatic parenchyma, cardiac muscle, gastrointestinal tract, bone or brain [1,2].
Chromosomal mutations appearing within PDGFRB gene represent as a common mode of pathogenesis for occurrence of myopericytoma and myofibroma. Cellular myofibroma and myopericytoma delineate SRF-RELA genetic fusion. Sporadic, infantile myofibroma, solitary myofibroma arising in adults, sporadic infantile myofibromatosis, lesions configuring myopericytomatosis and conventional myopericytoma demonstrate alterations within PDGFRB gene. Besides, PDGFRB genetic mutation N666K may occur within myopericytomatosis. However, cells constituting conventional myopericytoma are devoid of aforesaid chromosomal mutation. Repetitive fusion of SRF-RELA gene may be encountered within cellular myofibroma or myopericytoma [1,2]. Myopericytoma arising within individuals with acquired immune deficiency syndrome may concur with Epstein Barr Virus (EBV) infection. A subset of solitary or multiple lesions of infantile myofibromatosis may appear as a familial disorder with an autosomal dominant mode of disease inheritance. Occasionally, the condition may express Commonly, myopericytoma and myofibroma represent as a painless, gradually progressive mass of extended duration. Predominantly emerging as a solitary lesion, multiple lesions configure myopericytomatosis or myofibromatosis confined to a specific anatomical region or diverse zones [2,3]an autosomal recessive mode of inheritance [2,3].
Frozen section examination exhibits perivascular aggregates and concentric dissemination of plump, spindle shaped, myoid cells. Grossly, myopericytoma confined to superficial sites appears as a well circumscribed, nodular lesion <2 centimeter diameter. Enlarged neoplasms are confined to deep seated soft tissue. Cut surface is firm and tan to grey/white [3,4]. Upon microscopy, myopericytoma configures nodular or lobular, well circumscribed lesions. Tumefaction is composed of bland, ovoid to spindle shaped myoid cells which characteristically demonstrate multi-layering and concentric cellular articulations circumscribing innumerable, miniature vascular structures [3,4]. Exhibiting variable cellularity, tumefaction appears as a cellular, solid lesion or a hypocellular neoplasm commingled with collagenous or myxoid areas. Innumerable vascular structures of variable magnitude and branching, hemangiopericytoma-like vascular configurations may be observed. A prominent fascicular articulation of tumour cells may appear [3,4]. Few myopericytomas exhibit degenerative alterations as symplastic nuclear atypia, hyalinised stroma or cystic alterations. Myopericytomatosis emerges as an exceptional, benign variant of myopericytoma configuring diffuse lesions. Characteristically, tumefaction occurs within superficial soft tissues wherein implicated adults delineate innumerable myopericytomatous nodules [3,4]. Myofibroma arises as a well circumscribed neoplasm with tumour nodules delineating a characteristic, distinctive biphasic pattern of tumour evolution [3,4].
Figure 1:Myopericytoma delineating centric zone of immature myoid cells surrounded by fascicles of spindle shaped myoid cells intermingled with branching vascular articulations (Source: Libre pathology).
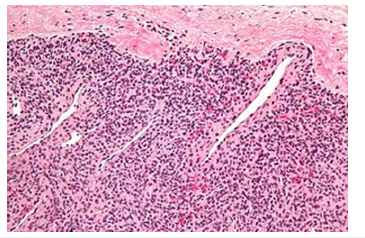
Tumour periphery is comprised of nodules and fascicles of myoid appearing cells intermingled with foci of variable hyalinization. Centric zone of the lesion is constituted of immature, plump, spindle shaped tumour cells with concurrent hemangiopericytoma-like, branching vascular structures. Neoplasm may depict haphazard or reverse zonation pattern [3,4]. Mitotic activity is variable. Cellular tumour zones may delineate focal necrosis and calcification. Infantile myofibroma may be comprehensively constituted of primitive, cellular zones and was previously designated as infantile hemangiopericytoma. Cellular zones of myofibroma morphologically simulate myopericytoma. Myofibromatosis is constituted of multiple myofibromas. Cellular myofibroma or myopericytoma with atypical features expounds elevated cellularity and solid configuration with focal infiltration into encompassing soft tissues. Mitotic activity is enhanced with >10 mitotic figures/10 high power fields. Tumour necrosis may be absent or exhibit areas of infarction. Cellular and nuclear pleomorphism is absent [3,4]. Malignant myopericytoma is constituted of ovoid to spindle shaped cells. Mitotic activity is elevated. Nuclear atypia and pleomorphism is significant. Tumour necrosis may be observed. Focal areas of intense perivascular distribution of tumour cells are observed, akin to benign myopericytoma [3,4] (Figures 1&2). Mitotic count is predominantly calculated from mitotically active areas, devoid of tumour necrosis. Mitosis may be quantified within 10 consecutive High Power Fields (HPF) upon 40x objective or 1 HPFx400=0.1734mm2 area wherein appropriate high power fields encompassing 1mm2 area is contingent to individual microscope (Tables 1 & 2).
Figure 2:Myopericytoma exhibiting centric zone of immature myoid cells encompassed within fascicles of spindle shaped myoid cells intermingled with branching vascular configurations (Source: Wikipedia).
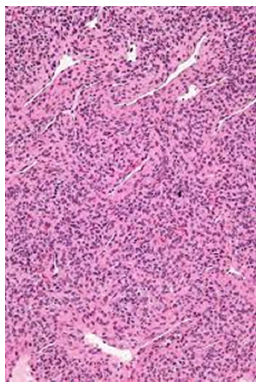
Table 1:Mitotic score of soft tissue sarcomas [3,4]. HPF: high power field

Table 2:Tumour necrosis associated with necrosis [3,4].

Tumour necrosis is appropriately evaluated upon cogent gross examination and categorized upon histological sections. Myopericytoma appears immune reactive to alpha Smooth Muscle Actin(α SMA), h-caldesmon or Muscle Specific Actin (MSA). Tumour cells appear immune non-reactive to desmin, CD34, S100 protein, keratin, myogenin, MyoD1 or Glial Fibrillary Acidic Protein (GFAP) [5,6]. Myopericytoma requires segregation from neoplasms as glomus tumour, angioleiomyoma, PEComa or infantile fibrosarcoma [5,6]. Myofibroma may be appropriately discerned upon cogent histological examination of obtained surgical tissue samples. Magnetic Resonance Imaging (MRI) exhibits predominantly superficial tumours. Well defined neoplasms configure myopericytoma and inadequately defined lesions articulate myopericytomatosis. Aforesaid conditions demonstrate intensely vascularized soft tissue masses with avid image enhancement. Areas of internal hemorrhage are frequently discerned [5,6]. Surgical eradication of the neoplasm is an optimal, recommended therapeutic strategy with superior clinical outcomes. Discernible alterations within PDGFRB gene is indicative of tyrosine kinase inhibition, a feature which may be beneficially adopted as a potential treatment strategy for myopericytic neoplasms [5,6]. Majority of myopericytomas and myofibromas are devoid of tumour reoccurrence. A subset of myofibromas or myopericytomas may delineate atypical features and emerge as diffuse, hypercellular lesions with elevated mitotic activity and a capacity for soft tissue infiltration. However, aforesaid features may not adversely influence therapeutic outcomes [5,6]. Malignant myopericytoma expounds an aggressive clinical course. Multicentric myofibroma or infantile myofibromatosis emerges as a life-threatening condition demonstrating tumour associated mortality [5,6].
References
- Yuan J, Li J, Dong Z, Wei X, Zhanbo W (2023) Primary hepatic myopericytoma coexisting with multiple cystic hepatic lesions: A case report. World J Surg Oncol 21(1): 15.
- Zhang L, Kang M, Kossard S, Kurosh P (2023) Myopericytomatosis: A rare entity mimicking other vascular tumours and malformations. Australas J Dermatol 64(2): 255-259.
- Sandoval V, Halstuch D, Huynh M, Bret W, Nicholas P (2023) Myopericytoma of the ureter Incidental finding of a benign slowly growing tumor. Urol Case Rep 47: 102362.
- Oshiro H, Shimizu Y, Nakayasu R, Noriaki U, Satsuki A, et al. (2023) Myopericytoma in the corpus cavernosum of the penis: A case report of a rare disease. IJU Case Rep 6(3): 181-184.
- Jang LC, Yoo KC (2023) Spontaneous deep vein thrombosis of the upper arm due to an intravascular myopericytoma: A case report and literature review. Medicine (Baltimore) 102(49): e36566.
- Kim JW, Kwon HJ, Kim MJ, Chang HH (2023) Two cases of cutaneous myopericytoma: A rare perivascular tumor with distinct histologic and immunohistochemical features. Ann Dermatol 35(Suppl 2): S361-S363.
© 2024. Anubha Bajaj. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






