- Submissions

Full Text
Novel Approaches in Cancer Study
Hypoxic Cancer Cells Protect Themselves Against Damage: Search for a Single-Cell Indicator of This Protective Response
Polina Schwartsburd* and Konstantin Aslanidi
Institute of Theoretical and Experimental Biophysics, Russia
*Corresponding author:Polina Schwartsburd, Institute of Theoretical and Experimental Biophysics, Russia
Submission: May 16, 2023Published: June 06, 2023

ISSN:2637-773XVolume7 Issue4
Abstract
Tumor hypoxia is accompanied by resistance to many damaging therapeutic effects. This effect is often attributed to the loss of the ability of normal and benign tumor cells to adapt their metabolism to hypoxia, whereas cancer cells, on the contrary, acquire the ability to survive and avoid an immune response under hypoxic conditions. Moreover, cancer cells use hypoxia to reprogram their metabolism and induce antioxidant protection, in particular by increasing the production of antioxidant molecules [NAD(P)H and glutathione], which protect hypoxic cancer cells from the damaging effects of free radicals. However, hypoxic cancer cells may differ one from another by the efficiency of protection against damage; an indicator is required to assess this efficiency. The goal of our work is the search for such a single-cell endogenous indicator. For this, we measured endogenous NAD(P)H fluorescence in single hypoxic cancer cells during their UV-induced damage [NAD(P)H photodegradation], as well as determined their ability to restore NAD(P)H fluorescence intensity after the damage/(photo)degradation. Most hypoxic cancer (but not normal) cells were experimentally found to be capable of partially restoring the damage with different efficiencies. Some hypoxic cancer cells possess increased resistance to UV-induced damage. Such cells retained the ability to partially restore NAD(P)H intensity even after 5-6 cycles of repeated damage. Our studies enable recommending the NAD(P)H regenerative response observed in hypoxic tumor cells as a prognostic indicator to assess their potential resistance to damage. This indicator can be useful in choosing new antitumor capabilities of specifically destroying cancer cells with increased resistance to damage.
Keywords:Cancer resistance; Hypoxia, Injury; NAD(P)H; Single-cell indicator
Introduction
Cancer cells differ from normal cells in various biological ways, including the reprogramming of their metabolic pathways, as well as in creating a protective microenvironment around themselves [1]. As a result, cancer cells gain advantages in survival and avoidance of an immune response during hypoxia, whereas normal cells and benign tumors do not possess such adaptive abilities [2]. Moreover, hypoxic cancer cells are capable of protecting themselves from damage and death, in particular due to the induction and enhancement of antioxidant protection supported by such molecules as glutathione and NAD(P)H [3]. As a result, hypoxic cancer cells acquire additional resistance to anticancer radiotherapy, which reduces the survival rate of cancer patients. The interaction between cancer cells and their hypoxic microenvironment plays a key role in maintaining the increased resistance of hypoxic cancer cells to damage. At the same time, hypoxia often acts in conjunction with other stressors, such as excess lactate and glucose deficit. Therefore, it is not surprising that hypoxic tumor cells are able to survive when glucose or lipids are available but lose this ability at their deficit in the tumor microenvironment [4]. In other words, cancer cells may differ functionally depending on the availability of oxygen and substrates required for their survival in vivo; therefore, to assess the efficiency of their protection from damage, it was necessary to develop a single-cell indicator. The search for such an endogenous indicator was the goal of this work.
Can NAD(P)H fluorescence be used as an indicator of the resistance of single hypoxic cancer cells to damage?
The reduced form of Nicotinamide Adenine Dinucleotide Phosphate [NAD(P)H] is found in various living cells and acts as an endogenous fluorophore. NAD(P)H is also a critical reducing agent necessary for cancer cells to maintain a stress-survival response under hypoxic conditions. This effect can be explained by the fact that hypoxia stimulates the expression and activation of protective genes required for the synthesis of antioxidants [Glutathione, Thioredoxin and NAD(P)H], thereby reducing the negative effects of stress by increasing the removal of free radicals (FR) during hypoxia [3,5]. Moreover, hypoxia contributes to an increase in the level of NAD(P)H in living cells and tissues, especially in hypoxic cancer cells [6,7]. Summing up all these properties of NAD(P)H, it can be assumed that the fluorescence intensity of NAD(P)H and its changes in response to UV-induced photodegradation (or damage) can be used as a potential indicator of the resistance of hypoxic cancer cells to FR-induced damage. To examine this assumption, we investigated the NAD(P)H fluorescent response in various hypoxic cells during their damage/photodegradation, as well as the potential ability of hypoxic cancer cells to restore NAD(P)H intensity after damage (i.e., in the absence of UV light). Measurements were performed on zejdela ascitic hepatoma (ZAH) cells.
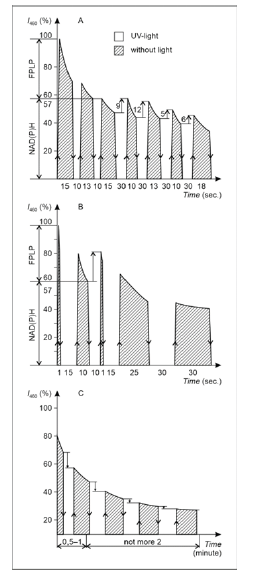
After the cells were quickly extracted from rat intraperitoneal cavity, they were placed in conditions of acute hypoxia artificially created in vitro. For this, they were placed between the slide glass and the cover glass, thoroughly sealed with paraffin to block the access of oxygen, and then exposed to ultraviolet light, causing cell fluorescence. In the fluorescence band where NAD(P)H fluoresces (400-470nm), lipid peroxidation products (FPLP) also fluoresce. NAD(P)H fluorescence can be separated from FPLP, which, as compared with NAD(P)H, have an increased sensitivity to photodegradation [8]. It should also be noted that many aggressive ZAH cells contain increased amounts of lipid droplets (LDs) [9], consisting of 90% neutral lipids sensitive to peroxidation; therefore, only those areas of the cytoplasm that contained no LDs were used to measure fluorescence. It was in these areas of the cytoplasm that the NAD(P) photodegradation velocity [i.e., changes in NAD(P)H fluorescence intensity with a maximum of 460nm (I460) under the action of exciting light with λ=365nm] was measured. The photodegradation process could be stopped by turning off the exciting light and leaving the cells in the dark. When the exciting light was switched on again, the photodegradation process resumed, starting with the reduced I460 level reached in the first cycle of NAD(P)H fluorescence damage. Studying hypoxic ZAH cells, we found that the predominant majority of such cells were capable of I460 partial recovery under the dark conditions. In some ZAH cells, this ability to restore I460 could persist for quite a long time even after repeated 4-5-fold cycles of photodegradation, although the increase in I460 in each recovery cycle was small. An example of such a cell is given in (Figure 1A), where the amount of I460 partial recovery in each damage-recovery cycle is marked with vertical arrows. However, there were also cells capable of effectively restoring the intensity of NAD(P)H only after the first damage cycle but did not retain the ability to restore I460 in response to subsequent damage (Figure 1B). In other words, such cells exhibited only a short-term resistance to damage but were not able to protect themselves from repeated damage for a long time. Thus, we consider only a repetitive protective response of a hypoxic cancer cell (Figure 1A) -- not an increase in the intensity of I460 -- as an indicator of its increased resistance to damage. The reason for the observed quantitative differences in the increase of I460 in response to identical damage (Figure 1A & 1B) was not established.
Are NAD(P)H metabolism inhibitors targets for cancer cells?
One of the main functions of the altered cancer metabolism is the increased production of NAD(P)H [10], the recovery potential of which is required, in particular, to maintain the activity of key antioxidants that protect cells from free radical damage [11]. NAD(P)H is also a critically important reducing agent necessary for hypoxic cancers cells for their adaptation and survival after various damages. Therefore, inhibition of key enzymes that produce NAD(P) H can be a selective target capable of increasing the efficiency of radiation therapy, as well as other antitumor treatments. It has been found that isocitrate dehydrogenase (IDH1) is the most upregulated NAD(P)H-producing enzyme in glioblastoma and that its inhibition sensitizes glioblastoma to radiation in vitro and in vivo by inducing NAD(P)H-dependent cellular senescence. These results show that targeting IDH1 could be an efficacious and selective metabolic strategy to abrogate radiation resistance in glioblastoma by affecting numerous nodes of reductive biosynthesis [5]. Another way of reducing NAD(P)H in solid tumor is achieved by hypoxia-responsive micelles that deplete NAD(P)H and impair two antioxidant cascades responsible for elevated cancer cell death [12].
Several inhibitors in the NAD(P)H-dependent antioxidant system are also used, such as sulfasalazine (an inhibitor of glutathione) and chaetocin (an inhibitor of thioredoxin); both increase cancer cell death [12]. It has been found that glucose-lowering drug metformin exerts its effect through decreasing the cytosolic NAD/ NADH ratio [13]. Unexpectedly, when metformin combines with resveratrol, a synergistic anticancer effect in triple-negative breast cancer is observed [14]. These examples confirm the assumption that inhibition of NAD(P)H metabolism in cancer cells can improve the therapeutic efficacy of radiation and chemotherapy. However, the negative effect of hypoxia on other normal cells present in the tumor microenvironment cannot be ignored. For this reason, we investigated the protective resistance of single lymphocytes located in ascitic ZAH fluid. Unlike tumor ZAH cells (Figure 1A & 1B), hypoxic lymphocytes were found not to be capable of restoring I460 after damage (Figure 1C). Such changes can be considered as a reduced ability of lymphocytes to recover and survive after damage, which contributes to the suppression of the local immune response. Under such conditions, a heterogeneous population of tumor cells acquire additional advantages for survival and propagation under hypoxic conditions. This is primarily contributed to by hypoxic tumor cells with prolonged self-protection to chronic damage, the search for potential markers of which was the purpose of this study
Although the mechanisms by means of which NAD(P)H contributes to increased resistance of hypoxic cancer cells to FRinduced damage are not fully understood, the search for such individual cancer cells is of extreme importance. We propose such a single-cell indicator that can be of help in predicting the treatment, as well as in the search for and screening of new drugs that selectively act exactly on highly resistant tumor cells (Figure 1).
Figure 1: An illustration of how cancer-derived NAD(P)H autofluorescence and its recovery after NAD(P)H photodegradation can be used for rapid monitoring of potential hypoxia resistance in single cancer (Figure 1A & 1B) but not normal (Figure 1C) cells. Different living cancer cells including ZAH cells (Figure 1A-C) show two different groups of fluorescent components in the 440-470nm band: (1) fluorescent products of lipid peroxidation (FPLP) characterized by a high rate of UV-induced photodegradation and inability of hypoxic tumor cells to restore I460 in the dark after exposure to UV light (λ=365nm); (2) NAD(P)H fluorescence with a lower rate of UV photodegradation than FPLP. The NAD(P)H intensity is increased in hypoxic cancer cells, which are also able to restore I460 in the dark after UV photodegradation (the increase is shown by vertical arrows). The ordinate represents fluorescence intensity at λ=460nm (I460) in relative units. The abscissa indicates the exposure time in seconds (Figure 1A & 1B) and minutes (Figure 1C). Information about ZAH cells, their growth in vivo and the protocol for creating hypoxia for ZAH cells and preparing samples for microspectrofluorescence studies from them has been published earlier (for details, see Supplementary Information in [9]). The fluorescent spectra were measured with a microspectrofluorimeter assembled at the laboratory.
Conflicts of interest statement and authors declaration template
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome. We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing we confirm that we have followed the regulations of our institution concerning intellectual property.
Author Contributions
P.S. designed the concept, wrote the paper, and prepared the manuscript and the figure. K.A. assembled the laboratory microspectrofluorimeter and improved the sensitivity of fluorescence registration.
References
- Hompland T, Fjeldbo CS, Lyng H (2021) Tumor hypoxia as a barrier in cancer therapy: Why levels matter. Cancers 13(3): 499.
- Boutry J, Tissot S, Ujvari B, Capp JP, Giraudeau M, et al. (2022) The evolution and ecology of benign tumors. Biochim Biophys Acta Rev Cancer 1877(1): 188643.
- Samanta D, Semenza GL (2018) Metabolic adaptation of cancer and immune cells mediated by hypoxia-inducible factors. Biochim Biophys Acta Rev Cancer 1870(1): 15-22.
- Lisec J, Jaeger C, Rashid R, Munir R, Zaidi N (2019) Cancer cell lipid class homeostasis is altered under nutrient-deprivation but stable under hypoxia. BMC Cancer 19(1): 501.
- Wahl DR, Dresser J, Wilder-Romans K, Parsels DJ, Zhao SG, et al. (2017) Glioblastoma therapy can be augmented by targeting IDH1-mediated NAD(P)H biosynthesis. Cancer Res 77(4): 960-970.
- Georgakoudi I, Jacobson BC, Muller MG, Sheets EE, Badizadegan K, et al. (2002) NAD(P)H and collagen as in vivo quantitative fluorescent biomarkers of epithelial precancerous changes. Cancer Res 62(3): 682-687.
- Pan X, Zhao Y, Cheng T, Zheng A, Ge A, et al. (2019) Monitoring NAD(P)H by an ultrasensitive fluorescent probe to reveal reductive stress induced by natural antioxidants in HepG2 cells under hypoxia. Chem Sci 10(35): 8179-8186.
- Schwartsburd PM, Aslanidi KB (1991) Resistance of single tumor cells and their intracellular compartments to lipid peroxidation. Med Oncol Tumor Pharmacother 8(2): 57-61.
- Schwartsburd PM (2022) Lipid droplets: could they be involved in cancer growth and cancer-microenvironment communications? Cancer Communications 42(2): 83-87.
- Villette S, Deshayes PS, Bizet VC, Validire P, Heckly GB, et al. (2006) Ultraviolet-induced autofluorescence characterization of normal and tumoral esophageal epithelium calls with quantitation of NAD(P)H. Photochem Photobiol Sci 5(5): 483-492.
- Chen Y, Li Y, Huang L, Du Y, Gan F, et al. (2021) Antioxidative stress: Inhibiting reactive oxygen species production species production as a cause of radioresistance and chemoresistance. Oxid Med Cell Longev, Article ID: 6620306.
- Guo X, Liu F, Deng J, Dai P, Qin Y, et al. (2020) Electron-accepting micelles deplete reduced nicotinamide adenine dinucleotide phosphate and impair two antioxidant cascades for ferroptosis-induced tumor eradication. ACS Nano 14(11): 14715-14730.
- Madiraju AK, Erion DM, Rahimi Y, Zhang XM, Braddock DT, et al. (2014) Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature 510(7506): 542-546.
- Cheng T, Wang C, Lu Q, Cao Y, Yu W, et al. (2022) Metformin inhibits the tumor-promoting effect of low-dose resveratrol and enhances the anti-tumor of high-dose resveratrol by increasing its reducibility in triple negative breast cancer. Free Radic Biol Med 180: 108-120.
© 2023. Polina Schwartsburd. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






