- Submissions

Full Text
Novel Approaches in Cancer Study
Iodine-125 Seed Combined with Stent Placement and Transarterial Chemoembolization for Treatment of Hepatocellular Carcinoma with Portal Vein Tumor Thrombosis: A Systematic Review and Meta-Analysis
Wei Fan Sui*
Zhenjiang First People’s Hospital, China
*Corresponding author:Wei Fan Sui, Zhenjiang First People’s Hospital, China
Submission: October 31, 2022 Published: December 01, 2022

ISSN:2637-773XVolume7 Issue3
Abstract
Background: Portal vein tumor thrombus plays an important role in indicating the poor prognosis of hepatocellular carcinoma.
Purpose: To assess the safety and efficiency of iodine-125 seed combined with stent placement and transarterial chemoembolization for treatment of hepatocellular carcinoma with portal vein tumor thrombosis.
Materials and methods: We searched Cochrane library, PubMed, EMBASE, CNKI, Wang fang Data and CQVIP. We assessed the qualities of included studies. We analyzed the characteristic data, tested heterogeneity, explored heterogeneity and tested publication bias by software-Review Manger 3.5.
Result: Totally 7 clinical controlled trials were selected with the inclusion criterion. The results showed that the pressure of main portal vein after stent placement was significantly lower than no stent placement. The cumulative stent patency rates and survival rates in 6 and 12 months of iodine-125 seed combined with stent placement and transarterial chemoembolization were higher than conventional transarterial chemoembolization, transarterial chemoembolization combined with stent and 3-dimensional conformal radiotherapy.
z
Conclusion: For hepatocellular carcinoma patients with portal vein tumor thrombosis, iodine-125 seed combined with stent placement and transarterial chemoembolization was safety. The efficiency of iodine-125 seed combined with stent placement and transarterial chemoembolization were better than conventional transarterial chemoembolization, transarterial chemoembolization plus stent and transarterial chemoembolization combined with stent and 3-dimensional conformal radiotherapy.
Keywords:Hepatocellular carcinoma; Transarterial chemoembolization; Portal vein tumor thrombus; Stent; Iodine-125; Meta-analysis
Keywords:HCC: Hepatocellular Carcinoma; PVTT: Portal Vein Tumor Thrombus; TACE: Transarterial Chemoembolization; 3-DCRT: 3-Dimensional Conformal Radiotherapy; I125: Iodine-125
Introduction
Hepatocellular Carcinoma (HCC) is one of the most common malignancies both in world and China [1] and it is also the third frequent cause of cancer-related deaths in China [2]. Portal Vein Tumor Thrombus (PVTT) plays an important role in indicating the poor prognosis of HCC. PVTT can deteriorate liver function by decreasing blood supply to the normal liver, gastrointestinal bleeding and tumor recurrence [3]. Consequently, HCC with PVTT is regarded as unresectable. The standard first-line therapy in unresectable hepatocellular carcinoma was transarterial chemoembolization (TACE) [4]. However, the effect of conventional TACE in treating HCC with PVTT was limited. Moreover, conventional TACE exacerbated liver function failure in HCC with PVTT. Some research demonstrated that 3-dimensional conformal radiotherapy (3-DCRT) and 125I seeds prolonged the survival time in HCC with PVTT and did not make liver function worse [5,6]. However, 3-DCRT or 125I seed alone could not relieve the obstructed portal vein immediately. Stent placement in portal vein was proved safety and efficient in portal vein hypertension, which could delay the time of liver function failure and prolong the survival time in HCC with PVTT [7]. However, within a short period, portal vein might obstruct again because tumor thrombus re-grew into stent through the mesh of stent. With the development of medical technology, 125I seeds combined with stent placement and TACE was demonstrated safety and efficiency (Figure 1). It could prolong the survival time of HCC with PVTT and postponed the restenosis of the portal vein further [8-10]. However current clinical trails lack of large samples to demonstrate the clinical significance of 125I seed combined with stent placement and TACE in HCC with PVTT, and no systematic analysis on the safety and efficiency of 125I seeds combined with stent placement and TACE in HCC with PVTT. Hence, this study was aimed to carry out a meta-analysis to assess the safety and efficiency of 125I seeds combined with stent placement and TACE in HCC with PVTT.
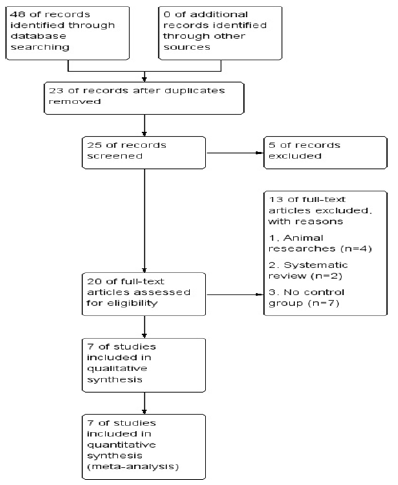
Figure 1: Flowchart shows flow of information through the different phases of systematic review toward a metaanalysis.
Materials and Methods
Search strategy
We conducted a comprehensive literature search both in English database including PubMed, Cochrane Library and Excerpt Medica Database and Chinese database including Chinese national Knowledge Infrastructure (CNKI), Wang fang Data and CQVIP up until 2019. We used the following search terms in the field for Title/Abstract and/or keywords: “hepatocellular carcinoma” and ‘‘transarterial chemoembolization’’ or ‘‘TACE’’ or “chemoembolization” and “Portal vein tumor thrombus” and “125I” and “stent”. All the data was available from published papers.
Study selection
The studies selected were required to meet the following inclusion criteria: 1) The original research. 2) The study participants were human. 3) The study had clinical results, such as, stent patency rates, survival rates, et al. 4) The study showed the clinical value of I125 seed combined with stent placement and TACE in HCC with PVTT. 5) The data could be extracted in study.
Data extraction and data quality assessment
Two doctors screened the titles and abstracts of potentially eligible studies independently and examined the full text articles to determine whether they could be included. One doctor independently extracted data, including author, country, publication year, design, treatment, patients’ number, et al. Then put the collected data into Review Management 5.3 to conduct meta-analysis. We assessed the quality of all included studies by Cochrane Collaboration’s tool [11].
Data analysis
We analyzed the data by Rev Man Manger 5.3. For all analyses,
P<0.05 was considered statistically significant. Heterogeneity was
assessed by using chi-square testing and I² statistics [12,13]. When
25%≤I²≤50%, it indicated low heterogeneity. When50%
Result
Search strategy
We selected 7 studies in this meta-analysis based on the criteria. Three studies were English [8-10] and four studies were Chinese [14-17].
Data extraction and data quality assessment
The extracted data contained information including the author, publication year, nation, study design, number of patients and therapies of experimental, control group. The characteristics of selected studies were induced in Table 1. Based on Cochrane collaboration’s tool including random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other bias, the results of selected studies were showed in Figure 2.
Figure 2: Risk of bias summary: review authors’ judgements about each risk of bias item for each included study. − high risk, +: low risk,?: unclear risk.
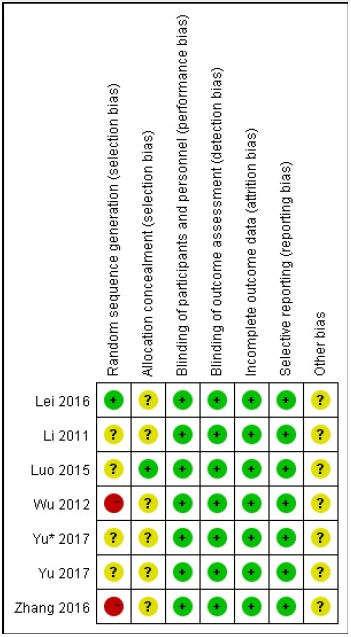
Table 1:The characteristics of included studies; NG: not given.

Data analysis
We compared pre-operation with post operation in the changes of main portal vein pressure (Figure 3). We found that the main portal vein pressure was decreased after stent placement(P<0.00001). The heterogeneity did not exist in this result (I2=0%). We did comparison in cumulative stent patency rates in 6 and 12 months between TACE+I125+stent with TACE+stent (Figure 4,5). The cumulative stent patency rates in 6 and 12 months of TACE+I125+stent were higher than TACE+stent and TACE+stent+3-DCRT(P<0.00001). The result indicated that therapies of stent without I125 seed and stent with 3-DCRT were easier to be obstructed by PVTT. The low heterogeneity existed in 6 months (I2=34%) and no heterogeneity existed in 12 months (I2=0%).
Figure 3: Forest plots of changes of main portal vein pressure.
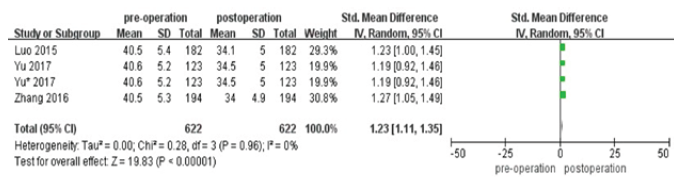
Figure 4: Forest plots of cumulative stent patency rates in 6 and 12 months.
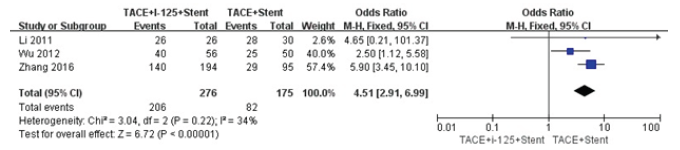
Figure 5: Forest plots of cumulative stent patency rates in 6 and 12 months.
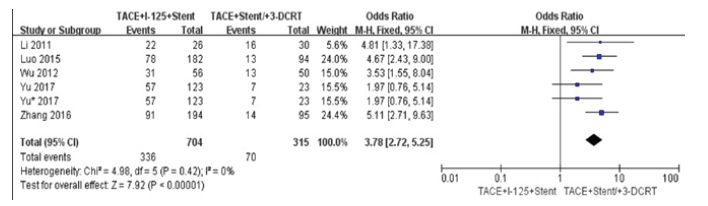
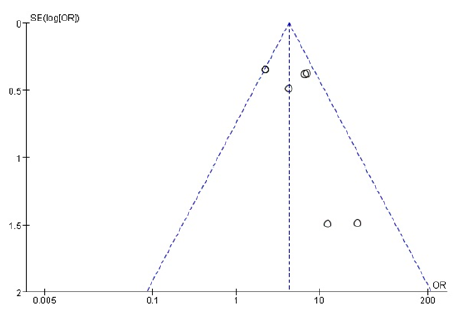
We did comparison in overall survival rate in 6 and 12 months among TACE+125I+stent, TACE+stent and TACE+stent+3-DCRT (Figure 6,7). The overall survival rate of TACE+125I+stent was higher than TACE+stent and TACE+stent+3-DCRT(P<0.00001). The results indicated that TACE+125I+stent could prolonger the survival time in HCC with PVTT than TACE+stent and TACE+stent+3-DCRT. The moderate heterogeneity existed in 6 and 12 months (I2=60%, I2=59%). The number of selected studies in overall survival rate in 6 months was not enough to explore the source of heterogeneity. consequently, we did subgroup analysis about the overall survival rates in 12 months (Figure 8). We found the heterogeneity of this meta-analysis was therapy(P<0.00001). The result also demonstrated that TACE+125I+stent in HCC with PVTT was better than TACE+stent and TACE+stent+3-DCRT. To assess publication bias, funnel plots regression was conducted and no publication bias was found in this meta-analysis (Figure 9).
Figure 6:Forest plots of survival rates in 6 and 12 months.
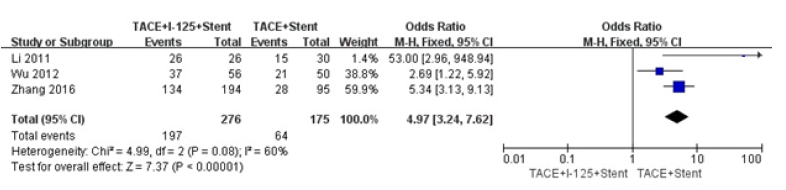
Figure 7:Forest plots of survival rates in 6 and 12 months.
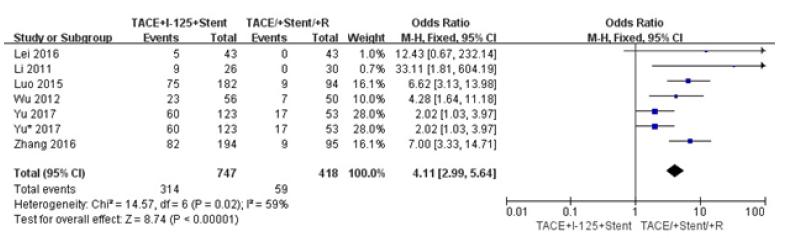
Figure 8:Forest plots of subgroup analysis about survival rates in 12 months.
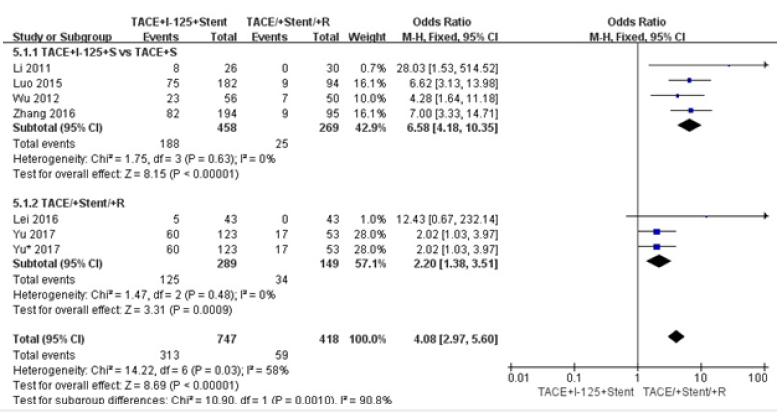
Figure 9:Funnel polt of included studies.
Discussion
Portal vein is nutrient vessel for liver, In HCC patients, the portal vein can be invaded by tumor metastasis and be obstructed by tumor thrombus. The drawbacks of portal vein occlusion are liver function failure and increased pressure of esophageal gastrointestinal bleeding, which are lethal for HCC patients. Hence, PVTT is recognized as one of the most significant signs for HCC. The effect of conventional TACE for HCC with PVTT was restricted because of indirect chemoembolization for PVTT. The effect of 3-DCRT alone was also restricted, because a radiation dose for PVTT was harmful to liver body [18]. Stent placement in portal vein was proved effective in decreasing portal vein pressure obviously [7]. However, the stent was obstructed easily by tumor thrombus re-grow through the mesh of stent. 125I seeds got close to tumor to delivery continuous low does irradiation, which restrained the ability to proliferate of tumor and induced apoptosis through damaging the DNA in the nuclei of the tumor cells. 125I seeds have been widely used in treating solid tumors like head and neck tumors, lung cancer, pancreatic cancer and prostate cancer [19-21]. In our meta-analysis, the therapy of TACE+125I+stent for HCC with PVTT was proved safety and efficient. To explore the heterogeneity, we found that the therapy of TACE+125I+stent significantly maintained the stent patency, extended the survival time and improved the prognosis of patients with HCC and PVTT. Compared with conventional TACE, stent in portal vein relieved the pressure of portal vein, which reduced the probability of lethal esophageal gastrointestinal bleeding and liver function failure. Furthermore, stent placement provided patients with opportunities for follow-up therapies. Compared with 3-DCRT, 125I seeds had a short radiation distance and highly accumulated radiation within tumor thrombus without serious damage to the surrounding tissue. Moreover, curative effect of 125I seeds was not affected by respiration motion. Some researchers showed that the combination of stent and 125I seeds was a good method for HCC with PVTT [22,23]. 125I seeds were prevented from loss and displacement by fixed in the site of tumor thrombus. It achieved fully covered radiation of tumor thrombus. Also, some researchers found that neointimal hyperplasia restenosis after stent placement could be counteracted by 125I seeds [24,25].
We should acknowledge the limitations of this meta-analysis. First, there were not enough prospective and high-qualities studies in selected studies that might uncover the clinical significance of TACE+125I+stent for HCC patients with PVTT. Second, with the limited number of selected studies, we cannot find more sources of heterogeneity. Third, the potential publication bias could not be ignored, although our result showed no significant publication bias. In summary, the therapy of TACE+125I+stent was better than conventional TACE, TACE+stent, and TACE+stent+3-DCRT in prolonging the survival time and improving long-term portal vein stent patency in HCC patients with PVTT.
References
- Torre LA, Bray F, Siegel RL, Ferlay J, Tieulent JL, et al. (2015) Global cancer statistics, 2012. CA Cancer J Clin 65(2): 87-108.
- Chen W, Zheng R, Baade PD, et al. (2016) Cancer statistics in China, 2015. CA Cancer J Clin 66(2): 115-132.
- Okuda K, Ohtsuki T, Obata H, Tomimatsu M, Okazaki N, et al. (1985) Natural history of hepatocellular carcinoma and prognosis in relation to treatment study of 850 patients. Cancer 56(4): 918-928.
- Cabibbo G, Tremosini S, Galati G (2014) Transarterial chemoembolization and sorafenib in hepatocellular carcinoma. Expert Review of Anticancer Therapy 14(7): 831-845.
- Sugiyama S, Beppu T, Ishiko T (2007) Efficacy of radiotherapy for PV and IVC tumor thrombosis in unresectable HCC. Hepato-gastroenterology 54(78): 1779-1782.
- Lee D S, Seong J (2014) Radiotherapeutic options for hepatocellular carcinoma with portal vein tumor thrombosis. Liver Cancer 3(1): 18-30.
- Yamakado K, Tanaka N, Nakatsuka A (1999) Clinical efficacy of portal vein stent placement in patients with hepatocellular carcinoma invading the main portal vein. Journal of Hepatology 30(4): 660-668.
- Chuan-Xing L, Xu H, Bao-Shan H (2011) Efficacy of therapy for hepatocellular carcinoma with portal vein tumor thrombus: chemoembolization and stent combined with iodine-125 seed. Cancer Biology & Therapy 12(10): 865-871.
- Luo JJ, Zhang ZH, Liu QX (2016) Endovascular brachytherapy combined with stent placement and TACE for treatment of HCC with main portal vein tumor thrombus. Hepatology International 10(1): 185-195.
- Yu TZ, Zhang W, Liu QX (2017) Endovascular brachytherapy combined with portal vein stenting and transarterial chemoembolization improves overall survival of hepatocellular carcinoma patients with main portal vein tumor thrombus. Oncotarget 8(7): 12108-12119.
- Higgins JPT, Altman DG, Gøtzsche PC (2011) The Cochrane collaboration’s tool for assessing risk of bias in randomized trials. BMJ 343: d5928.
- Higgins JPT, Thompson SG (2002) Quantifying heterogeneity in a meta‐analysis. Statistics in medicine. Stat Med 21(11): 1539-1558.
- Egger M, Smith GD, Schneider M (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109): 629-634.
- Zhen WL (2016) Efficacy of percutaneous transhepatic portal vein stent implantation combined with transcatheter arterial chemoembolization treatment in hepatocellular carcinoma patients with portal venous tumor emboli. China Journal of Modem Medicine 26(23): 111-114.
- Wu Lin lin, Luo jian jun, Yan zhi ping (2012) Comparative study of portal vein stent and TACE combined therapy with or without endovaseular implantation of iodiae-125 seeds strand for treating patients with hepatocellular carcinoma and main portal vein tumor thrombus. Chin J Hepatol 20(12): 915-919.
- Zhang zi han, Luo jian jun, Yan zhi ping (2016) Endovascular brachytherapy combined with stent placement and transarterial chemoembolization for treatment of hepatocellular carcinoma with main portal vein tumor thrombus. Hepatol Int 10(1): 185-195.
- Yu Tian zhu, Luo Jia Jun, Zhi Ping Yan (2017) Endovascular brachytherapy VS. sequential three-dimensional conformal radiotherapy for the treatment of main portal vein tumor thrombus: a comparative study. J Intervent Radiol 26(9):787-792.
- Dawson LA, Ten Haken RK, Lawrence TS (2001) Partial irradiation of the liver. Seminars in Radiation Oncology 11(3): 240-246.
- Johnson M, Colonias A, Parda D (2007) Dosimetric and technical aspects of intraoperative I-125 brachytherapy for stage I non-small cell lung cancer. Physics in Medicine & Biology 52(5): 1237-1245.
- Peretz T, Nori D, Hilaris B (1989) Treatment of primary unresectable carcinoma of the pancreas with I-125 implantation. Int J Radiat Oncol Biol Phys 17(5): 931-935.
- Ebara S, Katayama Y, Tanimoto R (2008) Iodine-125 seed implantation (permanent brachytherapy) for clinically localized prostate cancer. Acta Medica Okayama 62(1): 9-13.
- Zhang XB, Wang JH, Yan ZP (2009) Hepatocellular carcinoma invading the main portal vein: treatment with transcatheter arterial chemoembolization and portal vein stenting. Cardiovascular and Interventional Radiology 32(1): 52-61.
- Luo JJ, Yan ZP, Liu QX (2011) Endovascular placement of iodine-125 seed strand and stent combined with chemoembolization for treatment of hepatocellular carcinoma with tumor thrombus in main portal vein. Journal of Vascular and Interventional Radiology 22(4): 479-489.
- Amols HI (1999) Review of endovascular brachytherapy physics for prevention of restenosis. Cardiovascular Radiation Medicine 1(1): 64-71.
- Sidawy AN, Weiswasser JM, Waksman R (2002) Peripheral vascular brachytherapy. Journal of Vascular Surgery 35(5): 1041-1047.
© 2022. Wei Fan Sui. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






