- Submissions

Full Text
Novel Approaches in Cancer Study
Does HMBG1 Level Measured in Bronchoalveolar Lavage Liquid Contribute to Cytology?
Hülya Çiçek*
Department of Medical Biochemistry, Turkey
*Corresponding author:Hulya Cicek, Department of Medical Biochemistry, Turkey
Submission: June 10, 2022 Published: August 01, 2022

ISSN:2637-773XVolume7 Issue3
Abstract
Lung cancer is the leading cause of cancer-related death. Due to the poor prognosis, intensive research continues for early diagnosis and treatment. High Mobility Group Box (HMGB) proteins are nonhistone nuclear proteins with very different functions in the cell. HMGB1 can be released into the extracellular matrix, where it performs important functions in inflammation and carcinogenesis, such as promoting angiogenesis, preventing apoptosis, and promoting tissue invasion and metastasis. Patients who underwent bronchoscopy due to lung pathologies in Private Medical Park Gaziantep Hospital Thoracic Surgery Clinic were included in the study. Age, gender, bronchoscopy biopsy, cytology results, and clinicopathological evaluation results were obtained by scanning the patient files. Samples were obtained from the lavage fluid taken for Bronchoalveolar (BAL) cytology from patients who underwent bronchoscopy due to lung pathologies. After cytological evaluation, the remaining samples were stored at -80 °C. HMGB1 levels were measured from these samples [1]. Statistical analyzes were performed using SPSS for Windows 15.0 software. Before the study, approval was obtained from the SANKO University Clinical Research Ethics Committee. A total of 95 patients, 33 (34.7%) female, and 62 (65.3%) males, with a mean age of 59±15.2 (Range 17-86) were included in the study. When the final pathology results were evaluated together with clinical findings, 29 (30.5%) patients were diagnosed with primary lung cancer, 12 (12.6%) with metastatic lung cancer, 7 with COPD, and 47 with pneumonia. There was no statistically significant difference between the clinicopathological diagnosis groups in terms of hmgb1 level measured in bronchoalveolar lavage fluid (p=0.306). Clinicopathological diagnosis groups were grouped as malignant or benign. Benign lesions were detected in 54 (56.8%) patients, and malignant lesions were found in 41 (43.2%) patients. In terms of malignant and benign lesions, the level of hmgb1 measured in the bronchoalveolar lavage fluid was not statistically significant (p=0.146). When cytology was used to differentiate the lesions, 88 (92.6%) were reported as malignant and 7 (7.4%) as benign [2]. It was not statistically significant in terms of cytological results and hmgb1 level measured in bronchoalveolar lavage fluid (p=0.819). The sensitivity of cytology was 17%, the specificity was 100%, and the accuracy was 74.7%.
Methods such as endobronchial biopsy, bronchial lavage, transbronchial needle aspiration biopsy, and cytological brushing performed during bronchoscopy are used in the diagnosis of lung cancer. In addition to the studies arguing that the additional benefit of bronchial lavage cytology in the diagnosis is not very significant, there are also studies with the opposite view. In recent years, many studies have shown that there is a relationship between HMGB1 and cancer development. However, HMGB1 has no clear significance in the diagnosis and prognosis of cancer yet. Early diagnosis of NSCLC is very important in reducing the mortality of the disease. Therefore, the search for an ideal marker for early diagnosis continues. In our study, HMGB1 levels were measured in bronchoalveolar lavage fluid, but its diagnostic value was not determined.
Introduction
Bronchoalveolar Lavage (BAL) is a minimally invasive procedure performed with a fiberoptic bronchoscope and requires wedging within a selected bronchopulmonary segment (target site). BAL cellular analysis and cultures from the lower respiratory tract provide valuable information and insight into immunological, inflammatory, and malignant processes at the alveolar level [3]. BAL is a safe diagnostic procedure useful for differentiating fibrotic lung disorders and excluding malignancy and infection. BAL differential cell count can be affected by many factors, including the BAL procedure and the technical aspects of performing and analyzing this biological fluid (handling of lavage during bronchoscopy, lung region of BAL, and slide preparation). High Mobility Group Box (HMGB) proteins are non-histone nuclear proteins that have very different functions in the cell [4]. There is increasing evidence for the role of HMGB1 in cancer progression, angiogenesis, invasion, and metastasis. In the nucleus, HMGB1 plays an important role as a DNA-binding protein to maintain nucleosome structure and an architectural transcription factor that regulates gene expression. However, HMGB1 can be released into the extracellular matrix, where it exerts important functions in inflammation and carcinogenesis, such as promoting angiogenesis, preventing apoptosis, and promoting tissue invasion and metastasis. The HMGB1 protein is found in the nuclei of both cancer and normal cells. HMGB1 overexpression has been shown in many cancer types such as bladder cancer, colorectal cancer, and renal cell cancer in our previous studies. In these studies, performed on patient serum samples, higher serum Hmgb1 levels were detected compared to the control group. Hmgb1 levels in body fluids were studied. We showed that the levels of Hmgb1 measured in the urine were different between patients with bladder cancer, urinary infections, and the control group. This study aims to investigate whether Hmgb1 levels measured in BAL will contribute to improving the results of Bronchoalveolar Lavage (BAL) cytology used in the diagnosis of lung pathologies.
Patients and Method
Patient selection
Patients who underwent bronchoscopy due to lung pathologies in Private Medical Park Gaziantep Hospital Thoracic Surgery Clinic were included in the study [5]. All patients underwent standard pre-procedural evaluation for disease staging and prognosis. Age, gender, bronchoscopy biopsy, cytology results, and clinicopathological evaluation results were obtained by scanning the patient files.
Obtaining samples
Samples of lavage fluid taken for Bronchoalveolar (BAL) cytology were obtained from patients who underwent bronchoscopy due to lung pathologies. Samples remaining after cytological evaluation were stored at -80°C. HMGB1 levels were measured from these samples.
Measurement of BAL HMGB1 levels
BAL HMGB1 levels were quantitatively measured with Cloud- Clone brand commercial kits Catalog Number SEA399Hu according to the manufacturer’s instructions (Wuhan USCN Business Co., Ltd, Hubei/China). Analysis was performed using the Double-antibody Sandwich enzyme immunoassay technique. All concentration/ absorption graph curves of the Human HMGB1 test and calculations related to the results were made in the program Biotek_ELx808 (Winooski, Vermont, USA). The sensitivity of the test was 28.3pg/ ml and the detection range were 62.5-4000pg/mL [6]. Precision coefficients of variation within and between tests were found to be 8.3% and 9.1%, respectively. Before the study, approval was obtained from the SANKO University Clinical Research Ethics Committee. All participants in the study were informed about the study and written consent forms were obtained. It worked by the Declaration of Helsinki.
Statistical analysis
Statistical analyzes were performed using SPSS for Windows 15.0 software. The conformity of the variables to the normal distribution was examined using visual (histogram and probability graphs) and analytical methods (Kolmogorov-Smirnov/Shapiro- Wilk tests). Conditions with a p-value above 0.05 in the Kolmogorov- Smirnov test were accepted as a normal distribution. Since BAL HMGB1 did not show normal distribution, the differences between malignant and benign groups were investigated with the Mann- Whitney U test, which is a non-parametric test. Differences between clinicopathological patient groups were investigated with the Kruskal-Walli’s test. Diagnostic decision-making properties of BAL HMGB1 levels were analyzed by Receiver Operating Characteristics (ROC) curve analysis to determine the patient and control groups. In the evaluation of the area under the curve, the cases where the Type 1 error level was below 5% were interpreted as the diagnostic value of the test was statistically significant.
Result
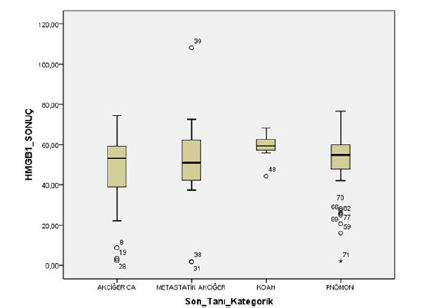
A total of 95 patients, 33 (34.7%) female, and 62 (65.3%) males were included in the study. The mean age of the patients was 59±15.2 (December 17-86). There was no significant age difference between male and female patients (p=0.052). The general characteristics of the patients are shown in Table 1. When the final pathology results were evaluated together with clinical findings, 29 (30.5%) patients were diagnosed with primary lung cancer, 12 (12.6%) patients with metastatic lung cancer, 7 patients with COPD, and 47 patients with pneumonia. There was no statistically significant difference between clinicopathological diagnosis groups in terms of HMGB1 level measured in bronchoalveolar lavage fluid (p=0.306, Figure 1). Clinicopathological diagnosis groups were grouped as malignant and benign. Benign lesions were detected in 54 (56.8%) patients and malignant lesions were detected in 41 (43.2%) patients. HMGB1 level measured in bronchoalveolar lavage fluid was not statistically significant in terms of malignant and benign lesions (p=0.146). When cytology was used to differentiate the lesions, 88 (92.6%) were reported as malignant and 7 (7.4%) as benign. It was not statistically significant in terms of cytological results and HMGB1 level measured in bronchoalveolar lavage fluid (p=0.819). The sensitivity of cytology was 17%, the specificity was 100%, and the accuracy was 74.7%. When the diagnostic value of the HMGB1 level measured in the bronchoalveolar lavage fluid is investigated by ROC analysis, it is seen that the measured values are below the ROC curve (Figure 2). This graph shows that the HMGB1 level measured in the bronchoalveolar lavage fluid is useless in the differential diagnosis of lung lesions.
Table 1:
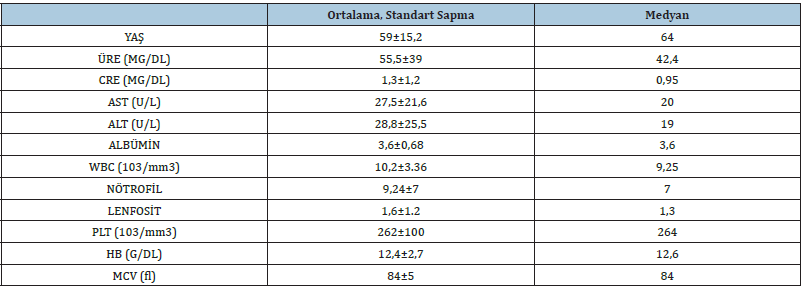
Figure 1:
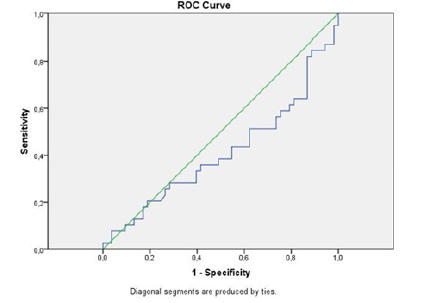
Figure 2:
Discussion
In the diagnosis of lung cancer, methods such as endobronchial biopsy, bronchial lavage, transbronchial needle aspiration biopsy, and cytological brushing are used during bronchoscopy. In addition to studies that argue that the additional benefit of bronchial lavage cytology in the diagnosis is not very significant, there are also studies with opposing views. In a single center, a large-scale study evaluating 455 patients, the diagnostic value of bronchial cytology was found to be as low as 15%. In the study of Dursun E et al. [7] the sensitivity of bronchial lavage was 16.6%, and the sensitivity of endobronchial biopsy pathological examination was 81.1%. However, the researchers stated that although the diagnostic value of a cytological examination is low, it may be valuable in the group of patients who do not have endobronchial lesions or who are not suitable for biopsy. The sensitivity of bronchial lavage was found at a relatively low level of 16.6%. The sensitivity of pathological examination of endobronchial biopsy was found 81.1%. Bronchial lavage cytology was found to be positive for malignancy in 1 out of 76 patients who received both bronchial lavage and biopsy in the same procedure and 5 out of 8 patients who could not have a biopsy due to some restrictions. At the end of this study, the researchers stated that although the diagnostic sensitivity of cytology samples was low, it was important because it was the only way to obtain bronchoscopy samples from patients without endobronchial lesions, patients who did not accept biopsy, and patients with high bleeding risk.
Due to the low sensitivity of BAL in the literature, there are various studies investigating tumor markers in BAL fluid and markers related to the tumor microenvironment. Wang H et al. [8] compared the concentrations of CEA, NSE, and CYFRA21-1 in BAL fluid with the control group and found them to be higher in the patient group. In this study, CEA levels were found to be high in patients with adenocarcinoma, NSE levels in small cell lung cancer, and CYFRA21-1 levels in patients with squamous cell lung cancer. Researchers have reported that looking at tumor markers in BAL fluid is more diagnostically meaningful when compared to tumor markers in serum. In the literature, there are various studies evaluating soluble contents in BAL fluid of patients diagnosed with lung cancer. The team of Kopinski et al. [9] focused on IL-27, which is a cytokine of multipotential function in cancer biology. These authors observed an elevated concentration of IL-27 in the BALF of patients with early-stage NSCLC corresponding with the Th1 profile. On the other hand, in the study by Naumnik et al. [10] they reported that B-cell-attracting chemokine 1 (BCA-1) and growth factor progranulin concentrations were higher in the BAL fluid of cancer patients compared to healthy individuals, which was a prognostic marker. Naumnik et al. [11] investigated many cytokines and chemokines in BALF, showing that they possess a prognostic significance. In their first studies, the differences in the concentration of TGF beta, VEGF, HMGB-1, IL-22, and IL-20 in BALF and serum between cancer patients and healthy volunteers were presented and correlated with the course of cancer. Recently they presented the results in which B lymphocyte chemoattractant, also known as B cell-attracting chemokine 1 (BCA-1), and the growth factor progranulin were elevated in the BALF of NSCLC patients when compared to healthy control subjects and were associated with cancer prognosis [10]. Similar findings concerned the BALF content of osteoprotegerin and a receptor activator of nuclear factor kappa B ligand (sRANKL).
Cancer development is a very complex dynamic process. In recent years, many studies have shown that there is a relationship between HMGB1 and cancer development. However, HMGB1 has no clear significance in the diagnosis and prognosis of cancer yet. In a study by Xia et al. [12] it was observed that HMGB1 levels detected by immunohistochemistry, RT-PCR, and Western blot techniques were higher in NSCLC patients compared to the healthy population. In a previous study, we compared serum Caveolin-1, HMGB1, and Endocan levels in patients with NSCLC and healthy individuals. In this study, we found that serum Caveolin-1 and HMGB1 levels were higher in patients with NSCLC, although they were not statistically significant when compared to healthy individuals, while Endocan levels were lower [13]. In this study, we compared the serum levels of Caveolin-1, HMGB1, and Endocan, which can be effective in the carcinogenesis steps, to patients with Non-Small Cell Lung Carcinoma (NSCLC) and healthy population. Caveolin-1, HMGB1, and Endocan serum levels were investigated using the ELISA method. Caveolin-1 and HMGB1 levels were higher in the patient group than in the control group, whereas the Endocan level was lower in the patient group than in the control group. But there was no statistical significance for the three parameters. In our study, we found no significant difference in terms of Endocan, Caveolin-1, and HMGB1 levels compared to a healthy population. Jakubowska K et al. [14] compared HMGB1 and TGF-beta levels in serum and BAL fluid of patients with NSCLC, Sarcoidosis, and healthy volunteers. They reported that serum HMGB1 levels were found to be higher in patients with NSCLC when compared with other groups. Researchers also reported a positive correlation between the HMGB1 level in BAL fluid and distant metastasis. Lung cancer is the most common cancer in the world and the leading cause of cancer-related death. Significant advances have been made in the diagnosis and treatment of lung cancer in recent years. Early diagnosis of NSCLC is very important in reducing the mortality of the disease. Therefore, the search for an ideal marker for early diagnosis continues. In our study, HMGB1 levels were measured in the bronchoalveolar lavage fluid, but its diagnostic value was not determined.
References
- https://www.who.int/news-room/fact-sheets/detail/cancer
- Pan C, Wang Y, Qiu MK, Wang SQ, Liu YB, et al. (2017) Knockdown of HMGB1 inhibits cell proliferation and induces apoptosis in hemangioma via downregulation of AKT pathway. J Biol Regul Homeost Agents 31(1): 41-49.
- Vijayakumar EC, Bhatt LK, Prabhavalkar KS (2019) High Mobility Group Box-1 [ HMGB1]: A potential target in therapeutics. Curr Drug Targets 20(14): 1474‐1485.
- Kang R, Chen R, Zhang Q, Hou W, Wu S, et al. (2014) HMGB1 in health and disease. Mol Aspects Med 40: 1-116.
- Wu L, Yang L (2018) The function and mechanism of HMGB1 in lung cancer and its potential therapeutic implications. Oncol Lett 15(5): 6799-6805.
- Girard P, Caliandro R, Seguin Givelet A, Lenoir S, Gossot D, et al. (2016) Sensitivity of cytology specimens from bronchial aspirate or washing during bronchoscopy in the diagnosis of lung malignancies: An update. Clinical Lung Cancer 18(5): 512-518.
- Dursun E, Yılmaz S, Keleş AN (2020) Diagnostic sensitivity of bronchial lavage cytology in lung malignancies. Dicle Medical Journal 47(4): 940-946.
- Wang H, Zhang X, Liu X, Liu K, Li Y, et al. (2016) Diagnostic value of bronchoalveolar lavage fluid and serum tumor markers for lung cancer. J Can Res Ther 12(1): 355-358.
- Kopiński P, Wandtke T, Dyczek A, Wędrowska E, Roży A, et al. (2018) Increased levels of interleukin 27 in patients with early clinical stages of non-small cell lung cancer. Pol Arch Intern Med 128(2): 105-114.
- Naumnik W, Panek B, Ossolińska M, Naumnik B (2019) B cell-attracting chemokine-1 and progranulin in bronchoalveolar lavage fluid of patients with advanced non-small cell lung cancer: new prognostic factors. Adv Exp Med Biol 1150: 11-16.
- Naumnik W, Płońska I, Ossolińska M, Nikliński J, Naumnik B (2018) Prognostic value of osteoprotegerin and sRANKL in bronchoalveolar lavage fluid of patients with advanced non-small cell lung cancer. Adv Exp Med Biol 1047: 1-6.
- Xia Q, Xu J, Chen H, Gao Y, Gong F, et al. (2016) Association between an elevated level of HMGB1 and non-small-cell lung cancer: a meta-analysis and literature review. Onco Targets Ther 9: 3917-3923.
- Sever ÖN, Çiçek H, Benlier N, Yildirim Z, Yildirim OA, et al. (2019) Prognostic role of serum HMGB1, endocan, and caveolin level in nonsmall cell lung cancer. J Mol Cancer 2(1): 15-17.
- Jakubowska K, Naumnik W, Niklińska W, Chyczewska E (2015) Clinical significance of HMGB-1 and TGF-β level in serum and balf of advanced non-small cell lung cancer. Adv Exp Med Biol 852: 49-58.
© 2022. Hülya Çiçek. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






