- Submissions

Full Text
Novel Approaches in Cancer Study
Towards a Holistic Approach in the Immunotherapy of Cancer
Ovidiu Farc* and Victor Cristea
Department of Immunology, Iuliu Hațieganu Medicine and Pharmacy University, Cluj-Napoca, Romania
*Corresponding author:Ovidiu Farc, Department of Immunology, Iuliu Hațieganu Medicine and Pharmacy University, Cluj-Napoca, Romania
Submission: July 22, 2022 Published: July 27, 2022

ISSN:2637-773XVolume7 Issue3
Abstract
The immune response against malignant tumors is performed through multiple immune cell types; each of them has been shown to be effective in its antitumor activity; this has inspired many therapeutic approaches such as adoptive cells transfers or stimulation through antitumoral antibodies or cytokines. The present opinion article is approaching the immune response in cancer from a holistic perspective, exploring the possibility that it may be used in integrated approaches, in cancer immunotherapy.
Keywords:Lymphocyte; Cancer; Integrated; Immunotherapy; Interle
Abbreviations:LT-Lymphocyte; NK-Natural Killer Lymphocyte; CD-Cluster of Differentiation; TH-T Helper Lymphocyte; PD-L1-Programmed-Death Receptor Ligand-1
Opinion
The immune response that the organism mounts against malignant tumors involves many types of immune cells, both innate, such as macrophages, neutrophils, eosinophils, or mast cells, or adaptive, such as different types of effectors, helper, T or B Lymphocytes (LTs). These cells infiltrate tumors in response to various danger signals, alterations in cell surface or antigenicity of tumor cells that constitute activation signals for each of them. The existing evidence highlights the potential of these many cell types of being strong antitumoral elements, sometimes completely eradicating tumors. It has been shown that macrophages [1], neutrophils [2], eosinophils [3], mast cells [4] or innate lymphoid cells such as NK [5], NKT [6] or γδ [7] lymphocytes may have a very strong tumoricidal effect under experimental conditions or in adoptive therapies to patients. Experimental evidence has shown that in addition to the well-known and recognized tumor killers (CD8+or Th1 LTs), immune subsets that were previously known as protumoral, such as Th2 [8], Th17 [9] or that were considered unimportant, such as B lymphocytes [10] or T follicular LTs [11] may also exert, under certain conditions, very strong antitumor effects, sometimes even stronger than Th1 LTs [12,13]. The result is that many of these immune cells are in use or in trials to be used as therapeutical means for cancer therapy [14]. However, it has been shown that these cells do not act always at their full antitumor potential, due to a complex of factors that are acting on them in tumors. In our recent paper [15], we have reviewed the factors that influence the immune cells to an antitumoral or, conversely, to a protumoral profile; it has been shown that when exposed to factors such as inflammatory or danger signals, specific chemokines, stimulatory or polarizing interleukins or specific antigens, immunocytes are stimulated towards am antitumor profile. In the meantime, following exposure to the immunosuppressive action of the tumor cells and the tumor microenvironment, which contains suppressive cytokines or exosomes, inhibitory molecules such as PD-L1 or CD47 or inappropriate physic-chemical conditions such as lactic acidosis, hypoxia or increased hydrostatic pressure, immunocytes are inhibited or even acquire protumoral properties [16].
Thus, not considering the conditions within tumors that influence immunocytes may lead to unsuccessful immunotherapies or adoptive transfers, due to the inhibition of the immunocytes involved. The factors that influence immune cells are important to be understood as possible causes of therapeutic failures, but also as possible opportunities to influence these cells for an antitumoral use. It has indeed been shown that cells like macrophages or mast cells are versatile cells that may be influenced through action on their TLR receptors to have an antitumoral profile [1,17]; in the meantime, lymphocytes may be directed through monoclonal or bispecific antibodies to the tumor cells to destroy them [18]. In the meantime, approaches that relieves the inhibitions on immunocytes, such as anti-PDL or anti CD47 therapies, have been shown to be extremely effective [19]. These facts, which concern the biology and function of immune cells, must be considered when a single immunocyte therapy, such as CD8+, NK or CAR T cell therapy, is desired. However, if a single immunocyte can be so successful in eradicating tumors (with the mentioned caveats), it seems reasonable to think about the possibility of considering the immune as a whole response, and to use its full power and effectiveness to fight tumors. The present work examines the fascinating possibility of going beyond the use of an immunocyte, an interleukin, a vaccine or an antitumoral antibody, or combinations of few of them, towards a holistic approach in which the entire immune response is used as a weapon in immunotherapy.
There were several recent studies that showed a certain degree of integration in the immune response, with the constitution of immune modules that comprise more than one type of immunocyte [20], which may allow integrated approaches to some of these modules or networks. To our knowledge, there is not yet any study that demonstrates an integrated behaviour of the immune response in tumors. To discuss about manipulating the immune response as a whole, a few facts should be considered. First, to influence the innate side of the tumoral immunity, the TLR receptors of immunocytes must be stimulated by TLR agonist molecules [17]. In addition, using TLR agonists may transform cold tumors into hot, immune-infiltrated tumors [21], by increasing the vascular adhesion and immunocyte influx into tumors, and by stimulating them; this approach may activate more than one innate subset, including dendritic cells. On the other hand, multiple experiments and trials have shown the spectacular effect that CAR-engineered LTs or the use of monoclonal or bispecific antibodies may have, leading to the eradication of even small nests of tumor cells [18]. These therapies, although very effective, also have drawbacks and failures, that may be overcome by the optimization of the approach in its basic mechanism [22] but failures may continue to happen, due to the environment in which these cells work, which is very suppressive [23]. By consequence, relieving the suppression of these cells through anti- PDL-1, anti- TIM-3, anti-CTLA-4, or other mechanism-based therapy proved to greatly extend the effectiveness of the therapies mentioned above. Although very successful, these therapies also have failures, which makes necessary to go deeper into the analysis of the nature of these inhibitions. Tumors are not sitting duck targets, they are tissues that undergo an aberrant development process which includes, as any tissue regeneration process, an immunosuppressive component [24]. It results that to get to the roots of any immunosuppression in tumors, one must consider the oncogenic process that causes immunosuppression, and, instead (or besides) addressing each of the suppressive mechanisms that were mentioned (which proved otherwise effective), to attack the oncogenic program that causes most of them. It has been shown in experiments that the inhibition of the oncogenic stimulation indeed blocks immunosuppression and changes the composition of the immune infiltrates [25]. Another phenomenon that is present in cancer is the imbalance of the Th subsets, mainly due to a dysfunction of the dendritic cell in cancer [26].
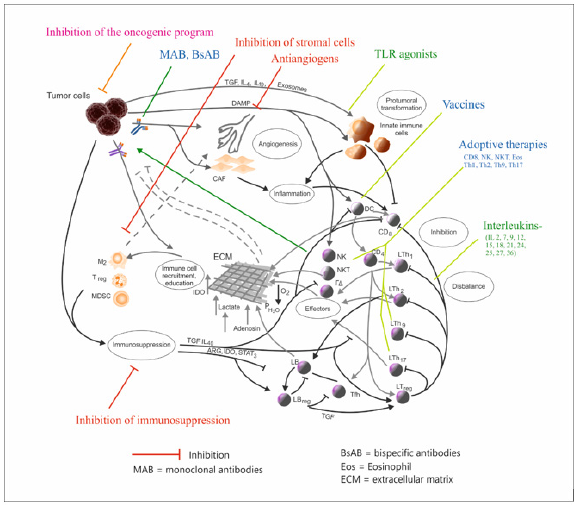
It has been suggested by some recent studies, that in a welldeveloped Th1-CD8+-NK-IFNγ environment, Th2 and Th17 LTs may be influenced to an antitumoral profile [27]. Polarizing interleukins such as IL-12, IL27 or IL-18 may be used to revert the Th imbalance in the tumor microenvironment, which has been shown to be effective. To summarize, an integrated approach which could involve the whole immune response in tumors would imply (Figure: 1): -stimulation of the main antitumor defense, which is the IFNγ-secreting network (Th1, CD8+, NK,NKT1,γδ-1 LTs); -modulation of inflammation through TLR agonists ; -inhibition of immunosuppression; a lot of means are used, but the analysis above showed that a reasonable approach is to attack the root of inhibitions which is the oncogenic program; the immunocytes would be freed from many of the conditions which make their activity difficult and ineffective. -optimizing Th balance by stimulating Th1 (using stimulatory or polarizing interleukins -IL-2, IL-12, IL27, IL-18, IL-7, IL-15) while leaving Th2 and Th17 in place, with the expectation that in these conditions they may exert an antitumoral role.
This combined approach would optimize both the innate and adaptive immune response (CD8+, Th and other subsets), by providing to cells their own stimuli, by inhibiting their suppression and by using or stimulating interactions between them, dealing with many of the setbacks of immunotherapies that were mentioned. It also results that a combined, holistic approach to the immune response must address the tumor microenvironment and its tumor cell-centered aberrant development program, with all its branches, which are in permanent interaction with the immune response (Figure 1). However, even such an approach which aims to touch virtually all of the compartments of the antitumor defense, would have to be developed in a personalized manner, measuring and keeping into account the magnitude of each of the factors that are involved: the immunosuppression, (by quantifying PD-L expression or other inhibitory molecules), the tumor antigens, inflammation and the different Th subsets ,which may be done through immune histochemistry, flow cytometry, computerized pathology [28] or by much simpler means such as cytokine profiles [29] ,or by combinations of them, so that peritumoral elements should be approached if their levels are increased. Thus, an optimal response could be obtained by the modulation of the different compartments of the tumor immunity. The network-based, integrated approaches are a field with great development potential, and they should be kept into account as a complement of the intelligent, mechanismbased approaches [18,22]. Of course, the proof of the mentioned considerations and approaches should be made in the appropriate experimental conditions.
Figure 1: The different immunotherapies that can be used in combined approaches, in the context of the tumor microenvironment interactions [15], modified, with permission]. M2, N2-M2 macrophages, N2 neutrophils; Treg-T Regulatory Lymphocyte; MDSC-Myeloid-Derived Suppressor Cell; Th-T Hepler Lymphocyte; IL-Interleukin; LB- B Lymphocyte; NK, NKT, γδ- NK, NKT, γδ lymphocyte; CAF-Carcinoma-Associated Fibroblasts; IDO- Indoleamin-2,3- Deoxygenase; ARG-Arginase; DAMP=Danger-Associated Molecular Pattern; TLR-Toll-Like Receptor; DC-Dendritic Cell; CD-Cluster of Differentiation.
References
- Pan Y, Yu Y, Wang X, Zhang T (2020) Tumor-associated macrophages in tumor immunity. Front Immunol 11: 583084.
- Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, et al. (2009) Polarization of tumor-associated neutrophil phenotype by TGF-beta: ‘N1’ versus ‘N2’ TAN. Cancer Cell 16(3): 183-194.
- Carretero R, Sektioglu IM, Garbi N, Salgado OC, Beckhove P, et al. (2015) Eosinophils orchestrate cancer rejection by normalizing tumor vessels and enhancing infiltration of CD8(+) T cells. Nat Immunol 16(6): 609-617.
- Varricchi G, Galdiero MR, Loffredo S, Marone G, Iannone R, et al. (2017) Are mast cells MASTers in cancer? Front Immunol 8: 424.
- Yang Q, Goding SR, Hokland ME, Basse PH (2006) Antitumor activity of NK cells. Immunol Res 36(1-3): 13-25.
- Terabe M, Berzofsky JA (2008) The role of NKT cells in tumor immunity. Adv Cancer Res 101: 277-348.
- Zhao Y, Niu C, Cui J (2018) Gamma-delta (γδ) T cells: Friend or foe in cancer development? J Transl Med 16(1): 3.
- Lorvik KB, Hammarström C, Fauskanger M, Haabeth OA, Zangani M, et al. (2016) Adoptive transfer of tumor specific Th2 cells eradicates tumors by triggering an in situ inflammatory immune response. Cancer Res 76(23): 6864-6876.
- Amicarella F, Muraro MG, Hirt C, Cremonesi E, Padovan E, et al. (2017) Dual role of tumour-infiltrating T helper 17 cells inhuman colorectal cancer. Gut 66(4): 692-704.
- Wouters MCA, Nelson BH (2018) Prognostic significance of tumor-infiltrating B cells and plasma cells in human cancer. Clin Cancer Res 24(24): 6125-6135.
- Gu Trantien C, Loi S, Garaud S, Equeter C, Libin M, et al. (2013) CD4+ follicular helper T cell infiltration predicts breast cancer survival. J Clin Invest. 123(7): 2873-2892.
- Martin Orozco N, Muranski P, Chung Y, Yang XO, Yamazaki T, et al. (2009) T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity 31(5): 787-798.
- Mattes J, Hulett M, Xie W, Hogan S, Rottenberg ME, et al. (2003) Immunotherapy of cytotoxic T cell-resistant tumors by T helper 2 cells: A eutaxon and STAT6-dependent process. J Exp Med 197(3): 387-393.
- Liu Y, Zeng G (2012) Cancer and innate immune system interactions: Translational potentials for cancer immunotherapy. J Immunother 35(4): 299-308.
- Farc O, Cristea V (2021) An overview of the tumor microenvironment, from cells to complex networks (Review). Exp Ther Med 21(1): 96.
- Burkholder B, Huang RY, Burgess R, Luo S, Jones VS, et al. (2014) Tumor-induced perturbations of cytokines and immune cell networks. Biochim Biophys Acta 1845(2): 182-201.
- Oldford SA, Haidl ID, Howatt MA, Leiva CA, Johnston B, et al. (2010) A critical role for mast cells and mast cell-derived IL-6 in TLR2-mediated inhibition of tumor growth. J Immunol 185(11): 7067-7076.
- Wong R, Pepper C, Brennan P, Nagorsen D, Man S, et al. (2013) Blinatumomab induces autologous T-cell killing of chronic lymphocytic leukemia cells. Haematologica 98(12): 1930-1938.
- Zhang X, Yang Z, An Y, Liu Y, Wei Q, et al. (2022) Clinical benefits of PD-1/PD-L1 inhibitors in patients with metastatic colorectal cancer: a systematic review and meta-analysis. World J Surg Oncol 20(1): 93.
- Bindea G, Mlecnik B, Tosolini M, Kirilovsky A, Waldner M, et al. (2013) Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity 39(4): 782-795.
- Liu YT, Sun ZJ (2021) Turning cold tumors into hot tumors by improving T-cell linfiltration. Theranostics 11(11): 5365-5386.
- Munks M, Levitsky V, Hill A, Knoetgen H (2015) Cytomegalovirus-specific CD8 T cells kill B16 melanoma cells in vivo when activated by bifunctional major histocompatibility class I-antibody fusion molecules (pMHCI-IgGs). J Immunotherapy Cancer 3(2): 237.
- Dunn GP, Old LJ, Schreiber RD (2004) The three Es of cancer immunoediting. Annu Rev Immunol 22: 329-360.
- Pesic M, Greten FR 2016) Inflammation and cancer: Tissue regeneration gone awry. Curr Opin Cell Biol 43: 55-61.
- Casey SC, Li Y, Felsher DW (2014) An essential role for the immune system in the mechanism of tumor regression following targeted oncogene inactivation. Immunol Res 58(2-3): 282-291.
- Ma Y, Shurin GV, Peiyuan Z, Shurin MR (2013) Dendritic cells in the cancer microenvironment. J Cancer 4(1): 36-44.
- Kryczek I, Banerjee M, Cheng P, Vatan L, Szeliga W, et al. (2019) Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood 114(6): 1141-1149.
- Schürch CM, Bhate SS, Barlow GL, Phillips DJ, Noti L, et al. (2020) Coordinated cellular neighborhoods orchestrate antitumoral immunity at the colorectal cancer invasive front. Cell 182(5): 1341-1359.
- Palucka AK, Coussens LM (2016) The Basis of oncoimmunology. Cell 164 (6): 1233-1247.
© 2022. Ovidiu Farc. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






