- Submissions

Full Text
Novel Approaches in Cancer Study
The Applications of Protein Nanomaterials in Cancer Therapies
Linjuan Wang1,2, Tingting Tang1,2, Kaiyue Zuo1,2, Xinjie Zhu1,2* and Wu Zhong3*
1Key Laboratory of Tropical Biological Resources of Ministry of Education, China
2Song Li’s Academician Workstation of Hainan University, China
3National Engineering Research Center for the Emergence Drugs, China
*Corresponding author:Xinjie Zhu and Wu Zhong, Key Laboratory of Tropical Biological Resources of Ministry of Education and National Engineering Research Center for the Emergence Drugs, China
Submission: June 20, 2022 Published: June 29, 2022

ISSN:2637-773XVolume7 Issue2
Abstract
Cancer serves as one of the main causes of death in global world, which has attracted tremendous attention due to high morbidities and mortalities. Currently, the traditional chemotherapies are still facing serious challenges, such as multidrug resistance, relapse and adverse events. What’s more, these risk factors will affect therapeutic efficacy. With the development of nanotechnologies, it provides great potential for cancer therapies ranging from non-targeting protein nanomaterials to nanobody with specific antigen recognition. In this mini review, we will mainly discuss the progress and approaches of protein nanomaterials being used to improve cancer therapeutic efficacy.
Keywords: Protein nanomaterials; Cancer therapies; Mitochondrial targeting; Nanobody
Mini Review
With the population aging and the development of society, cancer has become the main cause of death and seriously threated public health, which the burden of cancer has annually increased in the world [1,2]. According to GLOBOCAN 2020 reported by the International Agency for Cancer Statistics (IACS), there are approximately 19.3 million new cancer cases every year in 185 countries and territories. Among them, nearly 10 million patients will be dead from cancer. It estimates that the cases will increase 47 percent in 2040 compared with 2020 [3]. Conventional cancer therapies mainly contain chemotherapy, surgery, radiotherapy and immunotherapy. whereas these methods will produce some adverse events more or less due to off-target effect or inflammatory infections [4-7]. In recent decades, Innovations in nanotechnology have promoted drug delivery systems emerging as potential drug carrier candidates for cancer therapies. These nanomaterials could promote drug accumulations in tumor sites and improve through the Enhanced Permeability and Retention (EPR) effect. Additionally, nanomaterials based on protein have well biocompatibility and biological safety. In this work, we will further summary and discuss two aspect’s contents which mainly contain non-targeted protein nanomaterials and targeted protein nanomaterials in cancer therapies.
Non-Targeted Protein Nanomaterials
Nanomaterials have been widely applied to drug delivery systems. Nanomaterials based on protein have many advantages, such as well biocompatibility, degradability, solubility etc [8,9] (Figure 1). Our previous studies elucidated that the self-assembled protein nanoparticles GST-MT3 chelating with cobalt ions (Co2+) (coined as GST-MT3(Co2+)) was found, and it not only could target mitochondrion in vitro, but also could accumulate in tumor sites in vivo via EPR effect. Furthermore, GST-MT3(Co2+) could induce Reactive Oxygen Species (ROS) productions through Co2+ catalysis and reduce mitochondrial membrane potential then led to cell apoptosis. Additionally, it has well anti-tumor efficacy in 4T1 tumor bearing BALB/c mice and could synergistically prolong survival time via conjugating paclitaxel [10]. However, the molecular mechanisms of mitochondrial targeting remain unknown and whether it may have another targeting site is not yet clear besides EPR effect. Another protein nanomaterial was ferritin, it was firstly isolated from horse spleen in 1937 [11,12] and composed of 24 subunits, which reversibly self-assembled hollow and spherical protein nanoparticles with outer diameter of 12nm and inner diameter of 8nm [13-15]. Ferritin also has been widely applied to drug deliveries. Liang M et al. [13] demonstrated that ferritin loading high-dose doxorubicin (HFn-Dox) could accumulate in tumor sites via EPR effect and be degraded in lysosomes to release Dox then achieve cancer therapeutic efficacy [13]. Meanwhile, ferritin could interact with Transferrin Receptor (TFR1) (also called CD71) that acted as some tumor surface antigens, such as brain tumors and colorectal tumors, to achieve active targeting function rather than merely depend on EPR effect. Fan K et al. [16] elucidated that ferritin nanocarriers could bind with TFR1 to cross blood-brain barriers, and loaded drug molecules could inhibit glioma tumor growth, prolong the survival time of tumor-bearing mice [16].
Figure 1: Schematic diagram of nanomaterials for cancer therapies.
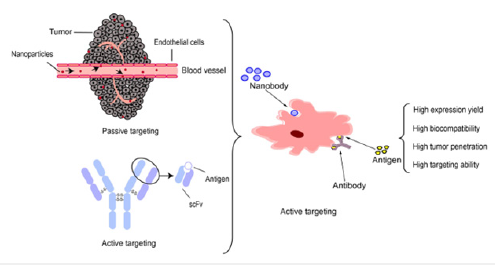
Targeted Protein Nanomaterials
Compared with passive targeting, active targeting therapies based on Antibody-Drug Conjugates (ADCs) are remarkably effective at eliminating some tumors through interaction with ligands. However, ADCs have limited therapeutic efficacy for some solid tumors due to poor penetration. Therefore, it is urgent to develop more smaller antibodies for cancer therapies. Hamers et al. [17] reported a camelid-derived antibody with an average molecular weight of 15kDa, a naturally occurring antibody without a light chain called a nanobody [17]. Single-domain antibodies (sdAbs) are individual heavy-chain antibody Variable Antigen-Binding Domains (VHHs), the smallest fragments of antibodies with binding capacity, that are characterized by small size, solubility, stability, and high penetration [18]. At present, single-domain antibodies have been proven to target intracellular proteins. With the development of biotechnology, target protein nanomaterials have evolved into the era of nanobodies. We have screened a high affinity nanobody, coined as 11-1, derived from the VHH of healthy Bactrian camels immunized with CD147 protein. 11-1 has well binding abilities for CD147 antigens in molecular level or in vitro assays. What’s more, it can target tumor sites then inhibit tumor growth in 4T1 breast tumor-bearing BALB/c mice [19]. Moreover, in the era of pandemic, nanobodies have also been used to treat COVID-19, and humanized single-domain antibodies effectively block viral infection by targeting spike protein beyond cancer therapies [20,21].
Discussion and Outlook
In this rapidly developed era of nanotechnology, the nanomaterials play important roles in the field of cancer therapies, which is expected to take great opportunities in the fight with cancer. Among them, nanomaterials based on protein have drawn widely attention due to high biocompatibility, solubility, degradable etc. However, one of biggest challenges it faces is to overcome tumor drug tolerance. Previous research elucidated that it may solve this problem through disrupting mitochondrial functions irreversibly [22-24]. We discovered novel self-assembly protein nanoparticle which could specially achieve mitochondrial targeting, and it mainly contained GST domain and MT3 domain. The latter one could encapsulate or chelate with cobalt ions then lead to mitochondrial member potential decrease and ROS produce through Co2+ catalysis. Eventually, it would induce cell apoptosis. Even so, a major dilemma still remains that non-targeted protein nanomaterials have more or less side effects due to “off-target”. To address this limitation, we have screened nanobody for CD147, termed 11-1 through phage display technologies. It has well tumor targeting ability for CD147 positive tumors. What’s more, compared with conventional antibody, there are many superior advantages, such as high expression, penetration, stability etc. Of note, nanobody almost couldn’t kill tumor cells by Antibody- Dependent Cellular Cytotoxicity (ADCC) effect. Taken together, although the non-targeted materials and targeted materials have great potential for cancer therapies, there are many incomplete molecular mechanisms to be addressed. We will further explore relate mechanisms in the future.
Funding
This work was supported by Fundamental Research Funds for Hainan University (KYQD(ZR)-21109).
References
- Bray F, Laversanne M, Weiderpass E, Soerjomataram I (2021) The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer 127(16): 3029-3030.
- Fidler MM, Bray F, Soerjomataram I (2018) The global cancer burden and human development: A review. Scand J Public Health 46(1): 27-36.
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3): 209-249.
- Minniti G, Goldsmith C, Brada M (2012) Radiotherapy. Handb Clin Neurol 104: 215-28.
- Lohcharoenkal W, Wang L, Chen YC, Rojanasakul Y (2014) Protein nanoparticles as drug delivery carriers for cancer therapy. Biomed Res Int 2014: 180549.
- Wang JJ, Lei KF, Han F (2018) Tumor microenvironment: recent advances in various cancer treatments. Eur Rev Med Pharmacol Sci 22(12): 3855-3864.
- Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, et al. (2007) Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol 2: 751-760.
- Luo Q, Hou C, Bai Y, Wang R, Liu J (2016) Protein assembly: versatile approaches to construct highly ordered nanostructures. Chem Rev 116(22): 13571-13632.
- Bai Y, Luo Q, Liu J (2016) Protein self-assembly via supramolecular strategies. Chem Soc Rev 45(10): 2756-2767.
- Zhu XJ, Li RF, Xu L, Yin H, Chen L, et al. (2019) A novel self-assembled mitochondria-targeting protein nanoparticle acting as theranostic platform for cancer. Small 15(2): e1803428.
- Fan K, Gao L, Yan X (2013) Human ferritin for tumor detection and therapy. Wiley Interdiscip Rev Nanomed Nanobiotechnol 5(4): 287-298.
- Tan H, Cheng D (2017) Using magnetoferritin nanoprobes for both nuclear and magnetic-resonance imaging. Nanomedicine (Lond) 12(1): 9-11.
- Liang M, Fan K, Zhou M, Duan D, Zheng J, et al. (2014) H-ferritin-nanocaged doxorubicin nanoparticles specifically target and kill tumors with a single-dose injection. Proc Natl Acad Sci U S A 111(41): 14900-14905.
- Plays M, Müller S, Rodriguez R (2021) Chemistry and biology of ferritin. Metallomics 13(5).
- Ahn B, Lee SG, Yoon HR, Lee JM, Oh HJ, et al. (2018) Four-fold channel-nicked human ferritin nanocages for active drug loading and ph-responsive drug release. Angew Chem Int Ed Engl 57(11): 2909-2913.
- Fan K, Jia X, Zhou M, Wang K, Conde J, et al. (2018) Ferritin nanocarrier traverses the blood brain barrier and kills glioma. ACS Nano 12(5): 4105-4115.
- Hamers CC, Atarhouch T, Muyldermans S, Robinson G, Hamers C, et al. (1993) Naturally occurring antibodies devoid of light chains. Nature 363(6428): 446-448.
- Khodabakhsh F, Behdani M, Rami A, Kazemi LF (2018) Single-domain antibodies or nanobodies: a class of next-generation antibodies. Int Rev Immunol 37(6): 316-322.
- Li R, Zhu X, Zhou P, Qiao Y, Li Y, et al. (2022) Generation of a high-affinity nanobody against cd147 for tumor targeting and therapeutic efficacy through conjugating doxorubicin. Front Immunol 13: 852700.
- Chi X, Liu X, Wang C, Zhang X, Li X, et al. (2020) Humanized single domain antibodies neutralize SARS-CoV-2 by targeting the spike receptor binding domain. Nat Commun 11(1): 4528.
- Saelens X, Schepens B (2021) Single-domain antibodies make a difference. Science 371(6530): 681-682.
- Marrache S, Pathak RK, Dhar S (2014) Detouring of cisplatin to access mitochondrial genome for overcoming resistance. Proc Natl Acad Sci U S A 111(29): 10444-10449.
- Chevalier A, Zhang Y, Khdour OM, Kaye JB, Hecht SM (2016) Mitochondrial nitroreductase activity enables selective imaging and therapeutic targeting. J Am Chem Soc 138: 12009-12012.
- Maiti KK, Lee WS, Takeuchi T, Watkins C, Fretz M, et al. (2007) Guanidine-containing molecular transporters: sorbitol-based transporters show high intracellular selectivity toward mitochondria. Angew Chem Int Ed Engl 46(31): 5880-5884.
© 2022. injie Zhu and Wu Zhong. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






