- About Us
- Information
-

The Author ensures that the research has been conducted responsibly and ethically with adherence to all relevant regulations. read more..
- For Authors
- For Reviewer
- Manuscript Guidelines
- Membership
- Publication Ethics
-
- Journals
- Reprints
- e-Books
- Videos
- Policies
- Contact Us
 COVID-19
COVID-19
-
Submissions

Full Text
Novel Approaches in Cancer Study
The Neoplastic Tonnage-Pleomorphic Xanthoastrocytoma
Anubha Bajaj*
Histopathologist in A.B. Diagnostics, India
*Corresponding author: Anubha Bajaj, Histopathologist in A.B. Diagnostics, New Delhi, India
Submission: May 20, 2022 Published: June 03, 2022

ISSN:2637-773XVolume7 Issue1
Abstract
- Kepes JJ, Rubinstein LJ, Eng LF (1979) Pleomorphic xanthoastrocytoma: a distinctive meningocerebral glioma of young subjects with relatively favorable prognosis. A study of 12 cases. Cancer 44(5): 1839-1852.
- Nesibe K, Tarik T (2013) Aggressive behaviour and anaplasia in pleomorphic xanthoastrocytoma: a plea for a revision of the current WHO classification. CNS Oncology 2(6): 523-530.
- Ebrahimi A, Korshunov A, Reifenberger G, Capper D, Felsberg J, et al. (2022) Pleomorphic xanthoastrocytoma is a heterogeneous entity with pTERT mutations prognosticating shorter survival. Acta Neuropathol Commun 10(1): 5.
- Chamberlain MC (2013) Salvage therapy with BRAF inhibitors for recurrent pleomorphic xanthoastrocytoma: a retrospective case series. J Neurooncol 114(2): 237-240.
- Lim S, Kim JH, Kim SA, Park ES, Shin YR, et al. (2013) Prognostic factors and therapeutic outcomes in 22 patients with pleomorphic xanthoastrocytoma. J Korean Neurosurg Soc 53(5): 281-287.
- Vu TM, Liubinas SV, Gonzales M, Drummond KJ (2012) Malignant potential of pleomorphic xanthoastrocytoma. J Clin Neurosci 19(1):12-20.
- Murray JC, Donahue DJ, Malik SI, Dzurik YB, Braly EZ, et al. (2011) Temporal lobe pleomorphic xanthoastrocytoma and acquired BRAF mutation in an adolescent with the constitutional 22q11.2 deletion syndrome. J Neurooncol 102(3): 509-514.
- Xiong J, Chu SG, Mao Y, Wang Y (2011) Pigmented pleomorphic xanthoastrocytoma: a rare variant and literature review. Neuropathology 31(1): 88-92.
- Dias-Santagata D, Lam Q, Vernovsky K, Vena N, Lennerz JK, et al. (2011) BRAF V600E mutations are common in pleomorphic xanthoastrocytoma: diagnostic and therapeutic implications. PLoS One 6(3): e17948.
- Schindler G, Capper D, Meyer J, Janzarik W, Omran H, et al. (2011) Analysis of BRAF V600E mutation in 1,320 nervous system tumours reveals high mutation frequencies in pleomorphic xanthoastrocytoma, ganglioglioma and extra-cerebellar pilocytic astrocytoma. Acta Neuropathol 121(3): 397-405.
Preface
Preface Pleomorphic xanthoastrocytoma is an exceptional, superficial, circumscribed, meningo-cerebral, astrocytic neoplasm. Pleomorphic xanthoastrocytoma was initially scripted as a distinctive astrocytoma by Kepes et al. in 1979 [1]. World Health Organization (WHO) categorizes pleomorphic astrocytoma as a grade II neoplasm. The significantly pleomorphic neoplasm with bizarre cytological features indicative of malignant biological behaviour is preponderantly associated with relatively superior prognosis. Localized reoccurrence may ensue wherein the classic, WHO grade II pleomorphic xanthoastrocytoma may reoccur as high grade, malignant ‘astrocytic’ glioma. The infrequent, aggressive, morphologically ‘anaplastic’ or malignant pleomorphic xanthoastrocytoma with anaplastic features is accompanied by specific clinical, radiological, and histological characteristics with significant mitotic activity exceeding >5 mitosis per 10 high power fields and demonstrates an inferior prognosis with an unpredictable biological course.
Disease Characteristics
Children and young adults are commonly incriminated although most neoplasms appear within 10 years to 30 years. No age of disease emergence is exempt [2,3]. Pleomorphic xanthoastrocytoma constitutes <1% of the primary astrocytic neoplasms. A specific gender or racial predilection is absent. The unique pleomorphic xanthoastrocytoma is predominantly situated within the superficial cerebral cortex or supra-tentorial region and adheres to superimposed leptomeninges wherein infiltration of the dura is exceptional. Commonly, the temporal lobe is implicated followed in frequency by the frontal lobe or parietal lobe [2,3]. Besides, the neoplasm may be located within the cerebellum, spinal cord, retina, thalamus, pineal gland or sellar region [2,3]. Appropriate tumour discernment exhibits a multi-centric tumefaction with several non-contiguous lesions. Ascertainment of histologically and genetically identical anaplastic variant emerging from a preceding site of classical pleomorphic xanthoastrocytoma is non challenging [2,3]. Malignant or anaplastic pleomorphic xanthoastrocytoma, preponderantly emerging as a relapsing neoplasm, is categorized as grade III or grade IV by the World Health Organization (WHO) [2,3]. Of obscure aetiology, it is posited that superficial pleomorphic xanthoastrocytoma is engendered from subpial astrocytes [2,3]. Tumefaction is postulated as a developmental, neuroglial tumour with prominent, glio-proliferative alterations accompanied with focal cortical dysplasia [2,3].
With an uncertain cell of origin, tumefaction is hypothesized to arise from ‘cerebral cortical astrocytic cells’ with superimposed pia mater, wherein aforesaid cells are immune reactive to glial markers as Glial Fibrillary Acidic Protein (GFAP) [2,3]. Apart from a glial genesis, tumefaction exhibits divergent differentiation as encountered with ‘mixed’ or ‘hybrid’ tumours exhibiting a neuronal or ganglionic component, wherein the cell of origin may digress from being purely astrocytic [2,3]. Thus, pleomorphic xanthoastrocytoma and aggressive subtypes may singularly be astrocytic neoplasms or may emerge as glioneuronal tumours [2,3]. Pleomorphic xanthoastrocytoma is associated with diverse central nervous system disorders such as neurofibromatosis type 1, focal cortical dysplasia, mesial temporal sclerosis or velocardiofacial syndrome [2,3].
Genetic Metamorphosis
Singular pleomorphic xanthoastrocytoma is genetically distinct from diffuse or infiltrating gliomas. Loss of Deoxyribonucleic Acid (DNA) upon chromosome 9 is a frequent regional chromosomal anomaly, discerned in an estimated 50% of neoplasms. Additionally, genetic gains and losses appear within chromosomes 3, 5, 20 and 22. Genomic gains emerge within loci of chromosomes 7 and 19. Associated genetic aberrations as translocations within chromosome 1, 15, 20 and 22 may be exemplified [3,4]. Infrequently, regional loss of genetic material within chromosomes 4, 6, 8p, 10p, 13, 17, 18 and 21 may occur. Chromosomal mutation of TP53 and amplification of Epidermal Growth Factor Receptor (EGFR) gene are exceptional. Besides, deletions or epigenetic inactivation of CDKN2A or CDKN2B genes appear inconsistent [3,4]. BRAF V600E genetic mutation is a contemporary, consistent genetic anomaly discovered in pleomorphic xanthoastrocytoma. Discernible, activating BRAF genetic mutations are beneficial in segregating pleomorphic xanthoastrocytoma from giant cell glioblastoma [3,4]. Additionally, proportionate BRAF V600E mutation may vary between adult and paediatric pleomorphic xanthoastrocytomas [3,4].
Clinical Elucidation
Superficial pleomorphic xanthoastrocytoma confined to the cerebral cortex demonstrates prolonged history of seizures preceding radiographic tumour detection [4,5]. Cogent, gradually progressive clinical symptoms appear as focal neurological deficits, visual disturbances, dizziness, headache and an infrequent intracerebral haemorrhage, symptoms which pertain to tumour location. The neoplasm is associated with intractable seizures. Frequently, tumefaction appears within the cerebral cortex of temporal lobe, demonstrates a distinctive cystic component, and predominantly engenders temporal lobe epilepsy. Exceptionally, pleomorphic xanthoastrocytoma can be asymptomatic [4,5]. A subset of neoplasms exhibits aggressive clinical behaviour with manifestations such as localized tumour reoccurrence or distant metastasis. An estimated one fifth of lesions may undergo malignant transformation [4,5].
Histological Elucidation
Grossly, the neoplasm is superficial, well circumscribed, frequently cystic and incriminates superimposed leptomeninges [4,5]. Pleomorphic xanthoastrocytoma exhibits significant cellular pleomorphism, an extensive reticulin framework and prominent, lipid-rich glial cells [4,5]. Upon microscopy, the superficial, compact neoplastic component is comprised of pleomorphic, spindle-shaped cells configuring a fascicular pattern. Mononuclear or multinucleated tumour cells with frequent nuclear inclusions and occasional cytoplasmic xanthomatous alterations can be discerned [5,6]. Perivascular cuffing with mature lymphocytes, disseminated eosinophilic, granular bodies and an abundant reticulin framework may be observed. Subjacent cerebral cortex may depict an infiltrative component of astrocytes [5,6]. Variable, focal hemorrhage and deposition of proteinaceous, granular degeneration can be delineated. Focal necrosis or mitotic activity is absent [5,6].
Microscopically, tumour perimeter is inadequately defined. Variable, pleomorphic histological features demonstrate a tumefaction composed of spindle-shaped or polygonal cells admixed with multinucleated giant cells and lipid-laden, xanthomatous astrocytes. Tumour cells configure fascicles or a storiform pattern, consequently simulating a mesenchymal tumefaction [5,6]. The atypical, pleomorphic, hyper-cellular neoplasm exhibits astrocytic or mesenchymal cells imbued with abundant cytoplasm. Pleomorphic nuclei generally exhibit nuclear inclusions and significant nuclear anisocytosis [5,6]. Xanthomatous cells incorporated with foamy cytoplasm are prognostic although encountered in roughly 25% of lesions [5,6]. Frequent, enlarged, bizarre, multinucleated giant cells impart the characteristic pleomorphic appearance. Intra-nuclear inclusions and eosinophilic granular bodies are consistently observed [5,6]. Focal, perivascular, or intra-tumoral aggregates of mature lymphocytes and occasional plasma cells may be exemplified [5,6]. Diffuse or patchy deposition of reticulin fibres circumscribing tumour cells is characteristic. Rosenthal fibres appear upon tumour periphery, indicative of reactive alterations within adjacent brain. Exceptionally, tumour cells may be permeated with pigment granules [5,6]. Cellular pleomorphism is frequent although mitotic figures and necrosis are absent, features which demarcate the neoplasm from malignant, infiltrating gliomas [5,6]. Tumefaction with malignant transformation or anaplastic pleomorphic xanthoastrocytoma exhibits significant mitotic activity ranging from <3 mitosis per 10 high power fields to up to 18 per 10 high power fields. Anaplastic pleomorphic xanthoastrocytoma or WHO grade III tumefaction lack a dense framework of reticulin fibres. Significant endothelial proliferation and focal necrosis is apparent. The anaplastic variant may exhibit pathognomonic morphological characteristics pertaining to a classical variant [5,6]. Anaplastic pleomorphic xanthoastrocytoma depicts enhanced mitotic activity exceeding >5 mitoses per 10 high power fields. Segregation from an epithelioid glioblastoma can be challenging [5,6]. Elevated mitotic activity exceeding ≥5 mitoses per 10 high power fields and focal necrosis with pseudo-palisading is accompanied by aggressive biological behaviour and inferior prognosis with decimated recurrence-free survival or overall survival. An anaplastic variant may exceptionally depict a distinctive rhabdoid component [5,6]. On ultrastructural analysis, tumour cells predominantly exhibit an astrocytic cellular origin and appear disparate from mesenchymal or meningothelial neoplasms. Tumour cells appear circumscribed by basal lamina. Neuronal features with microtubules and dense core granules can be discerned [5,6].
Immunohistochemistry
Pleomorphic xanthoastrocytoma is diffusely and intensely immune reactive to Glial Fibrillary Acidic Protein (GFAP), Olig-2, Sox2 and S100 protein, indicating a glial or astrocytic lineage. A neoplasm is variably immune reactive to diverse neuronal markers as synaptophysin, neurofilament, reticulin, class 3 β-tubulin and microtubule associated protein 2 (MAP2) [5,6]. Haematopoietic progenitor or vascular endothelial cell-associated antigen CD34 is enunciated in around 50% tumefaction [5,6]. Proliferation marker Ki-67 labelling index (MIB1) is generally below <1%. Ki-67 proliferative index and Proliferating Cell Nuclear Antigen (PCNA) are expounded in few tumour cells [5,6]. Reactivity to tumour suppressor gene p53 or chromogranin is variable, and the majority of tumefaction is immune non-reactive [5,6] (Figures 1-8).
Figure 1: Pleomorphic xanthoastrocytoma depicting spindle-shaped cells, lipid laden astrocytic cells admixed with multinucleated giant cells, focal lymphocytes and patchy reticulin fibres.
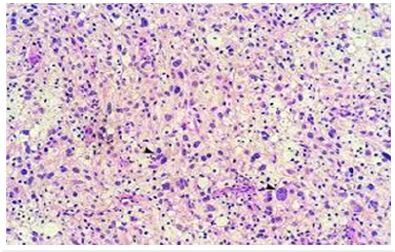
Figure 2: Pleomorphic xanthoastrocytoma demonstrating polygonal cells with abundant eosinophilic cytoplasm, intra-nuclear inclusions admixed with multinucleated giant cells peripheral Rosenthal fibres.
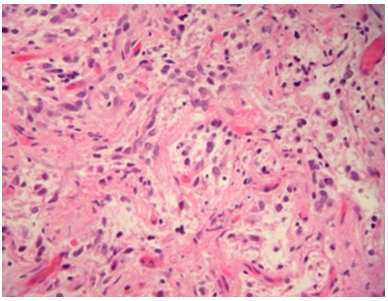
Figure 3:Pleomorphic xanthoastrocytoma delineating prominent, pleomorphic multinucleated giant cells admixed with polygonal cells with abundant cytoplasm with commingled lymphocytes.

Figure 4:Pleomorphic xanthoastrocytoma enunciating spindle-shaped cells intermingled with multinucleated giant cells, mature lymphocytes and sparse reticulin fibres.
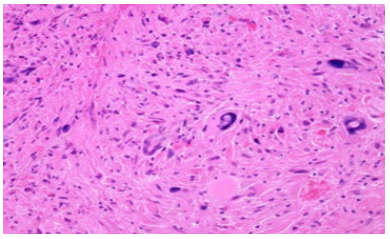
Figure 5:Pleomorphic xanthoastrocytoma exemplifying lipid-rich astrocytic cells admixed with multinucleated giant cells, polygonal cells, and circumscribing foci of compressed cerebral cortex.
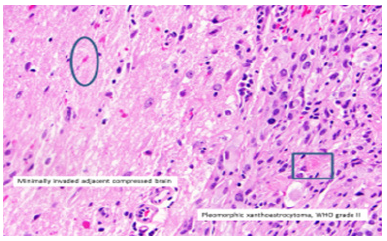
Figure 6:Pleomorphic xanthoastrocytoma exhibiting multinucleated giant cells admixed with polygonal cells with abundant cytoplasm, intra-nuclear inclusions, and few mature lymphocytes.
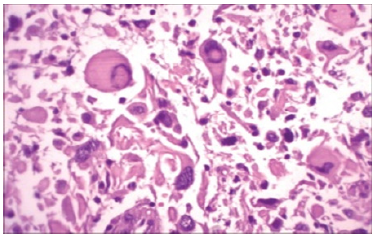
Figure 7:Pleomorphic xanthoastrocytoma depicting a fascicular arrangement with polygonal cells with abundant cytoplasm, mature lymphocytes and sparse reticulin fibres.
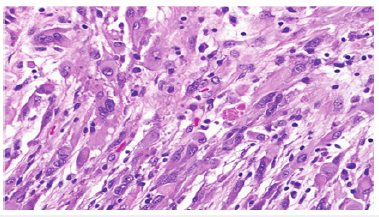
Figure 8:Pleomorphic xanthoastrocytoma-angiomatous variant demonstrating polygonal cells, multinucleated giant cells, perivascular cellular aggregates with lymphocytes and peripheral Rosenthal fibres.
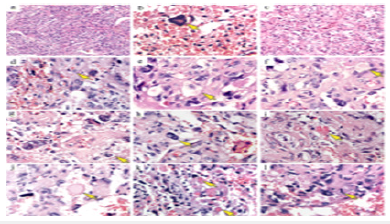
Differential Diagnosis
Pleomorphic xanthoastrocytoma requires a segregation from
1. Ganglioglioma or gangliocytoma which emerges as a
well-defined, “bubbly”, compact tumefaction, demonstrating
variably lobular architecture. Tumefaction is confined to the
cortical or subcortical region with minimal circumscribing
oedema. A neoplastic glial component is admixed with
proliferative, neoplastic ganglion cells. An astrocytic element
simulates fibrillary astrocytoma or pilocytic astrocytoma.
Mitosis is exceptional and tumour necrosis is usually absent.
Generally, tumefaction depicts a perivascular cuff of mature,
small lymphocytes. Besides, Rosenthal fibres and eosinophilic
granular bodies can be discerned. Micro-cysts, prominent
reticulin framework, focal calcification, vascular proliferation
with configuration of glomeruloid structures and desmoplasia
is enunciated, especially in neoplasms confined to the
subarachnoid space. Ki-67 proliferative index is minimal. Image
enhancement is observed upon Magnetic Resonance Imaging
(MRI). The tumour perimeter exhibits an elevated signal
intensity upon Fluid-Attenuated Inversion Recovery (FLAIR)
[7,8].
2. Desmoplastic infantile ganglioglioma occurs in young
children. Enlarged, multiple lesions are observed with
incrimination of dura [7,8].
3. Dysembryonic Neuroepithelial Tumour (DNET)
is a ‘bubbly’, intra-cortical lesion comprised of uniform,
oligodendroglioma-like cells intermingled with mucinous
substance. Specific glio-neuronal elements as cellular columns,
configured of axons layered with uniform, oligodendrogliomalike
cells commingled with floating neurons or cortical neurons
embedded in mucin pools, can be observed. Astrocytes
appear disseminated within tumour cells. Adjacent cerebral
cortex exhibits focal cortical dysplasia. Mitotic figures are
exceptional. Image enhancement upon contrast administration
is uncommon [7,8].
4. Oligodendroglioma is a diffuse, extensively cellular
neoplasm composed of oligodendrocytes with spherical
nucleus, distinct cellular outline, moderate to marked nuclear
atypia and clear cytoplasm. Few oligodendrogliomas are
comprised of cells with eosinophilic cytoplasm and focal,
perinuclear vacuolization. Abundant, delicate, acutely branching
capillaries or vascular articulations traverse the neoplasm.
Tumour calcification is prominent. Perineural, perivascular
or sub-pial aggregates of tumour cells, configuring secondary
structures of Scherer, are demonstrated. Few neoplasms may
depict eosinophilic granular bodies or a spongioblastoma-like
configuration [7,8].
5. Cystic meningioma is comprised of cells with elongated
processes and vacuolated cytoplasm, simulating micro-cysts.
Nuclear pleomorphism can be prominent. Mitotic activity is
minimal. An abundant vasculature is observed. Miniature nests
of classic meningothelial cells may appear. Micro-cystic foci are
mildly and diffusely immune reactive to carbonic anhydrase IX
[7,8].
6. Glioblastoma or giant cell glioblastoma is an extensively
anaplastic neoplasm composed of glial cells demonstrating significant nuclear atypia and pleomorphism. Focal
microvascular proliferation, glomerular tufts, and multiple
layers of endothelial cells with prominent mitosis and focal
necrosis can be discerned. Exceptionally, hypertrophic
proliferation of endothelial cells may ensue. Vascular
thrombosis and necrosis, especially with pseudo-palisading, is
associated with aggressive biological behaviour. Focal apoptosis
may abut zonal necrosis. Variable infiltrate of inflammatory
cells is exemplified. Mitotic activity is variable [7,8].
7. Malignant fibrous histiocytoma is associated with
divergent cellular morphology as spindle-shaped, spherical,
epithelioid, or pleomorphic cells. Significant cellular and
nuclear atypia with enhanced mitotic activity is observed.
Multinucleated giant cells or an infiltrate of inflammatory cells
appear admixed with the tumour cells [7,8].
8. Pilocytic astrocytoma is a biphasic neoplasm comprised
of compact, fibrillary segments, bipolar piloid processes as
Rosenthal fibres alternating with spongy, loose, micro-cystic
foci with commingled eosinophilic, granular bodies, cobweblike
processes, and tumour cells with spherical to elliptical
nuclei. Occasional multinucleated tumour giant cells are
exemplified [7,8].
Focal oligodendroglioma-like areas may be discerned along with foci of degenerative alterations. Vascular articulations appear glomeruloid. The well circumscribed, predominantly solid neoplasm displays restricted infiltration of abutting brain parenchyma or emerges as a cystic, mural nodule. Frequent neoplastic extension into subarachnoid space is observed [7,8].
Investigative Assay
Upon imaging, pleomorphic xanthoastrocytoma emerges as a solid, enhancing nodule frequently with a peripheral, eccentric, cystic component. Commonly cystic, the lesion appears as a cystic, mural nodule [9,10]. The peripheral, cortical neoplasm with leptomeningeal adherence may demonstrate a ‘dural tail’. Aforesaid manifestation appears as a reactive process rather than a true, exceptional neoplastic dissemination within the dura. Remodelling of superimposed or the adjacent skull is characteristic. The gradually progressive, superficial neoplasm displays variable vasogenic oedema [9,10]. Upon Computerized Tomography (CT) and magnetic resonance imaging (MRI), pleomorphic xanthoastrocytoma commonly emerges as a cystic lesion or a mural nodule which can be enhanced upon contrast administration. A neoplasm can be partially cystic with a minimal mass effect and depict image enhancement. The gradually progressive neoplasm may or may not be associated with peritumoral oedema. Upon CT, pleomorphic xanthoastrocytoma is characteristically hypodense or iso-dense, well demarcated or inadequately demarcated with minimal or absent circumscribing oedema. Tumour calcification is exceptional [9,10]. Upon T1 weighted (MRI), the solid tumour component is iso-intense to hypo-intense, whereas the cystic component exhibits an enhanced signal intensity. Leptomeninges are incriminated in a majority of instances [9,10]. Upon T2 weighted imaging and Fluid-Attenuated Inversion Recovery (FLAIR), cystic areas depict an enhanced signal intensity vis-à-vis cerebrospinal fluid due to an elevated protein content [9,10]. Upon administration of gadolinium contrast, vivid enhancement of images is observed [9,10]. Upon Digital Substraction Angiography, (DSA), pleomorphic xanthoastrocytoma appears avascular [9,10].
Therapeutic Options
Optimal, recommended treatment strategy is surgical extermination or gross total resection of the neoplasm, a manoeuver which is accompanied with a superior prognosis. Gross, comprehensive surgical resection of the neoplasm can alleviate seizures [9,10]. Subtotal tumour resection is associated with decimated progression free survival [9,10]. Tumefaction depicting BRAF mutations appear responsive to chemotherapy with targeted inhibitors wherein an activation paradox may considerably impair efficacy for a single agent chemotherapy. It is contemplated that combination chemotherapy may supplant resistance to single agent chemotherapy and appears beneficial in treating reoccurring pleomorphic xanthoastrocytoma [9,10]. Adjuvant radiotherapy may be adopted in individuals subjected to incomplete surgical resection or reoccurring neoplasms. Nevertheless, adjuvant radiotherapy or chemotherapy appear inefficacious as a pertinent therapeutic strategy [9,10]. Localized tumour reoccurrence may ensue. Tumour reoccurrence of preceding pleomorphic xanthoastrocytoma may occur wherein the reappearing neoplasm appears devoid of anaplastic features [9,10].
Aggressive tumefaction with unfavourable prognostic outcome may manifest cerebrospinal or leptomeningeal tumour dissemination [9,10]. Neoplasms confined to atypical locations or present with a challenging to access are accompanied with an inferior outcome [9,10]. Tumour magnitude and extent of surgical resection are significant prognostic factors influencing progression free survival. Also, an advanced age and spinal neoplastic dissemination at the initial tumour discernment are cogent factors influencing tumour prognosis [9,10]. Anaplastic or malignant transformation of classical pleomorphic xanthoastrocytoma is of variable duration and may occur up to 18 years following initial surgical intervention. Also, complete tumour remission is documented for up to 25 years. Anaplastic pleomorphic xanthoastrocytoma appearing de novo appears associated with decimated survival [9,10].
References
© 2022. Anubha Bajaj. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
Submit Query
PubMed Indexed Articles
- Kv3-Expressing Cells Present More Elaborate N-Glycans with Changes in Cytoskeletal Proteins, Neurite Structure and Cell Migration
PMID: 39736999 - Reliability of a Wearable Motion System for Clinical Evaluation of Dynamic Lumbar Spine Function
PMID: 36816092 - The Americans with Disabilities Act and Medication Assisted Treatment in Correctional Settings
PMID: 38770439 - Dendrimer-Based Nanomedicine (Paramagnetic Nanoparticle, Nanocombretastatin, Nanocurcumin) for Glioblastoma Multiforme Imaging and Therapy
PMID: 35237758 - Glioblastoma: Targeting Angiogenesis and Tyrosine Kinase Pathways
PMID: 32924014 - The Conflict in East Ukraine: A Growing Need for Addiction Research and Substance Use Intervention for Vulnerable Populations
PMID: 32363331
Track Your Article
Editor In Chief
Hirotada TSUJII
Ph.D in Agriculture from Faculty of Agriculture, Tohoku University

Maria Kuman
Research Professor, PhD, Holistic Research Institute

Jiexiong Feng
Professor, Chief Doctor, Director of Department of Pediatric Surgery, Associate Director of Department of Surgery, Doctoral Supervisor Tongji hospital, Tongji medical college, Huazhong University of Science and Technology
Muhammad Atiqullah
Senior Research Engineer and Professor, Center for Refining and Petrochemicals, Research Institute, King Fahd University of Petroleum and Minerals (KFUPM), Dhahran, Saudi Arabia

Ian James Martins
Fellow of International Agency for Standards and Ratings (IASR), Edith Cowan University, Sarich Neuroscience Research Institute

Thomas F George
Chancellor Emeritus / Professor Emeritus of Chemistry and Physics, University of Missouri–St. Louis
.jpg)
Jose Crisologo de Sales Silva
Ph.D in Science from the Federal University of Alagoas, UFAL, Brazil

Naglaa Sami Adbel Aziz Mahmoud
Assistant Professor in College of Architecture, Art and Design

Tong-Ching Tom Wu
Interim Dean, College of Education and Health Sciences, Director of Biomechanics Laboratory, Sport Science Innovation Program, Bridgewater State University

Dr. Jose Luis Turabian
Professor of numerous training courses in Family Medicine
Dariusz Jacek Jakóbczak
Assistant Professor, Department of Electronics and Computer Science

Önder Pekcan
Emeritus Professor of Physics, Kadir Has University, Turkey
Quick Links
-
 Editorial Board Registrations
Editorial Board Registrations
-
 Submit your Article
Submit your Article -
 Best Paper of the Volume
Best Paper of the Volume -
 Reprints
Reprints -
 Refer a Friend
×
Refer a Friend
×Refer a Friend
-
 Advertise With Us
×
Advertise With Us
×Advertise With Us
Our Recent Edition
Top Editors
-

Zhengcai Lou
Wenzhou Medical University, China
-

Ya Lie Ku
Fooyin University, Taiwan
-

Volkan Sarper Erikci
Saglik Bilimleri University, Turkey
-
Tomasz Karski
Vincent Pol University, Poland
-
.jpg)
Thamil Selvam
National Defence University of Malaysia, Malaysia
-

Tarik Baykara
Dogus University, Turkey
-

Steven Smith
Hope College, USA
-

Stanislav Grigoriev
Russian Academy of Sciences, Russia
-

Shi Zhou
Southern Cross University, Australia
-

Shewikar Farrag
Umm Al-Qura University, Saudi Arabia
-

Ray Marks
City University of New York, USA
-

Praveen K Maghelal
Khalifa University of Science & Technology, United Arab Emirates
-

Pipat Chooto
Prince of Songkla University, Thailand
-
Peng Yu
Hebei Normal University, China
-
Nawal Mohamed Khalafallah
Alexandria University, Egypt
-

N K Kishore
Indian Institute of Technology Kharagpur, India
-

Muzzalupo Innocenzo
Council for Agriculture Research and Analysis of Agri Economy (CREA), Italy
-

Muhammad Atiqullah
King Fahd University of Petroleum and Minerals, Saudi Arabia
-
Mohd Azlan Mohd Ishak
Universiti Teknologi MARA, Malaysia
-

Mohamed A Rashed
King Abdulaziz University, Saudi Arabia
-

Maurice E Morgenstein
University of Oregon, USA
-

Martin Sweatman
University of Edinburgh, Scotland
-
.bmp)
Maria Kuman
University of Tennessee, USA
-
.jpg)
Manuel Velasco
Central University of Venezuela, Venezuela
-
.png)
Majid Monajjemi
Islamic Azad University Central Tehran Branch, Iran
-
.jpg)
Luisetto Mauro
Tourin University, Italy
-

Lloyd Arthur Jenkins
Teaching & Public Speaking, Spain
-
Leonardo Milella
Paeditric Hospital "Giovanni XXIII", Italy
-

Katerina Chryssou
General Chemical State Laboratory , Greece
-

Kanakis Dimitrios
University of Nicosia, Cyprus
-

Jose Luis Clua Espuny
Universidad Miguel Hernández de Elche, Spain
-

John Korstad
Oral Roberts University, USA
-

Jinliang Zhang
Beijing Normal University, China
-

Irina Koretsky
Howard University, USA
-
Ian James Martins
Edith Cowan University, Australia
-

Hamid Yahiya Hussain
Dubai Health Authority, UAE
-

Gundu HR Rao
University of Minnesota, USA
-
GP Karmakar
Indian Institute of Technology Kharagpur, India
-

Ghassan George Haddad
Serhal Hospital, Lebanon
-
.jpg)
George Thomas
University of Missouri-St. Louis , USA
-

George Gregory Buttigieg
University of Malta, Malta
-

Fumihiko Hinoshita
National Center for Global Health and Medicine, Japan
-

Freida Pemberton
Molloy College, USA
-

Francisco Welington de Sousa Lima
Federal University of Piauí, Brazil
-

Florian Bert
Krankenhaus Nordwest Hospital, Germany
-

Fedor Lisetskii
Belgorod State University, Russia
-
.png)
Fathi Habashi
Laval University, Canada
-

Dora Alicia Cortes Hernandez
Cinvestav-Unidad Saltillo, Mexico
-
.png)
Daniel Kinem
UPMC Hamot Neuroscience Institute, USA
-

Conxita Mestres Miralles
Ramon Llull University, Spain
-
Barry Kraynack
White Bear Associates, LLC, USA
-

Arkady S Voloshin
Lehigh University, USA
-

Alireza Heidari
California Southern University, USA
-
.png)
Alex Guskov
Institute of Solid State Physics of RAS, Russia
-

Alan Diego Briem Stamm
University of Buenos Aires, Argentina
-
Ahmed Nasr Ghanem
Mansoura University, Egypt
-

Afaf K El Ansary
King Saud University, Saudi Arabia
-

A Bernardes
University of Coimbra, Portugal
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 





























.jpg)






