- Submissions

Full Text
Novel Approaches in Cancer Study
Current Therapeutics and Future Perspectives for Treatment of Advanced BRAF V600 mutant Melanoma
Tiantian Zhang1, Zhe Wang2, Claire Malhotra3, Holly Yin2 and Yan Xing3*
1Toni Stephenson Lymphoma Center, Department of Hematology and Hematopoietic Stem Cell Transplantation, Beckman Research Institute, City of Hope, CA, 91010, USA
2High Throughput Screening Core, Department of Share Resources, Beckman Research Institute, City of Hope, Duarte, CA, 91010, USA
3Department of Medical Oncology and Therapeutics Research, City of Hope, Duarte, CA, 91010, USA
*Corresponding author: Yan Xing, Department of Medical Oncology and Therapeutics Research, City of Hope, Duarte, CA, 91010, USA
Submission: March 15, 2022 Published: April 08, 2022

ISSN:2637-773XVolume7 Issue1
Abstract
The landscape of advanced melanoma therapies has undergone a dramatic transformation over the last decade. The better characterization of the Mitogen-Activated Protein Kinase (MAPK) pathway led to the development of BRAF and MEK inhibitors (BRAFi and MEKi), such as vemurafenib and trametinib, which have shown substantial improvement in patient response and survival rate. To further address the rapid resistance developed following BRAFi monotherapy, combined BRAFi and MEKi doublets were developed and have represented the standard of care for targeted therapy of advanced melanoma patients. The deeper understanding of immune cells and their interactions with tumor cells prompted the development of Immune Checkpoint Inhibitors (ICIs) such as ipilimumab and nivolumab, which have shown tremendous clinical benefit and led to the emergence of BRAFi+MEKi+ICI triplet treatment. In this article, we review the evolvement of treatment approaches for advanced BRAF V600 mutant melanoma and discuss the unmet needs and challenges to be addressed by future studies.
Keywords: Advanced melanoma; BRAF V600 mutation; Targeted therapy; Immunotherapy; Triplet therapy; Sequence of systemic therapies; Clinical trials
Abbreviations: MAPK: Mitogen-Activated Protein Kinase; FDA: Food and Drug Administration; CTLA-4: Cytotoxic T lymphocyte Antigen-4; PD-1: Programmed Cell Death Protein-1; OS: Overall Survival; PFS: Progression Free Survival; ORR: Objective Response Rate; CRR: Complete Response Rate; DOR: Duration of Response; LAG-3: Lymphocyte-Activation Gene-3; GM-CSF: Granulocyte Macrophage Colony-Stimulating Factor; T-VEC: Talimogene Laherparepvec, ICI: Immune Checkpoint Inhibitors
Introduction
The last decade has witnessed a dramatic revolution in novel treatment strategies for metastatic melanoma with BRAF V600 mutations. BRAFi and MEKi have shown striking efficacy through targeting BRAF and MAPK pathway mediators (e.g., MEK1 and MEK2), and combinations of these two therapies (BRAFi and MEKi doublets) have also been approved by the Food and Drug Administration (FDA) [1]. The advent of ICIs, including antibodies against cytotoxic T lymphocyte antigen-4 (CTLA-4) and programmed cell death protein-1 (PD-1), have provided another approach in disinhibiting T cell function and enhancing their anti-tumor immune response, rendering them promising in melanoma treatment. With ICIs, the treatment landscape of advanced BRAF V600 mutant melanoma further evolves with the emergence of the triplet treatment as first line treatment, which is composed of BRAFi, MEKi and ICIs. In this paper, we briefly review the current established and emerging treatments for BRAF-mutated advanced melanoma, particularly immunotherapy followed by BRAFi+MEKi doublet therapy, and triplet therapy, and emphasize the areas where unmet needs and uncertainties remain to be solved in the future.
Molecular Features and Therapeutic Rationale of Melanoma
Efforts in discovering driver mutations in melanoma started in the 1980s. However, it was not until 2002 when, through a systematic genetic approach, an activating mutation in BRAF, which is a serine threonine protein kinase in the RAS/RAF/MEK/ERK pathway, had been found harbored in nearly 70% of melanomas. Interestingly, ~90% of BRAF mutations are missense V600E/K mutations [2], which lead to a consequential activation of MEK and ERK signaling and result in a dysregulation of cell growth. Moreover, BRAF mutations have been found to associate with complex mutational profiles in malignant melanoma, including genomic aberrations of CDKN2A, CDK4 and TERT. For instance, while the CDK2NA gene encodes p16 protein which inhibits CDK4 and CDK6, concurrent mutations of BRAF and loss of functional p16 have been detected in the majority of melanomas. Given the established pathological role of BRAF V600 mutations and MEK activation in melanoma, targeted therapies with BRAFi (e.g., vemurafenib, dabrafenib, and encorafenib) and MEKi (e.g., cobimetinib, trametinib and binimetinib) have gained tremendous interest in the last decade.
Melanoma cells express not only immunogenically specific proteins, such as MART-1, tyrosinase, and gp-100, but also abundant neoantigens likely due to UV-related carcinogenesis, all of which induce a robust T cell immune response [3]. CTLA-4 and PD-1, which are expressed on activated T cells, interact respectively with B7 and PD-L1 which can cause a reduction in T cell function. Via blocking these interactions, ICIs have been shown to elicit remarkable antitumor responses and have revolutionized the treatment of many cancers, including advanced melanoma.
Frontline Therapies of Melanoma
In this portion, we will provide a summary of the common melanoma treatment modalities (Table 1), followed with review of targeted therapy, immunotherapy, triplet therapy and sequence of systemic therapies in advanced melanoma treatment.
Table 1: Melanoma treatment modalities.
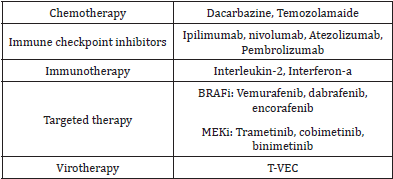
Targeted therapy: BRAF/MEK inhibition
In 2011, vemurafenib became the first BRAFi to be approved by the FDA for treatment of BRAF V600 mutant melanoma, followed by dabrafenib and encorafenib. These therapies all demonstrated improved survival outcomes in advanced melanoma treatment in comparison to standard chemotherapy such as dacarbazine, an alkylating agent [4]. More recently, Pfizer has developed a potent, selective, and highly brain-penetrant BRAF inhibitor PF-07284890 (ARRY-461) which is undergoing clinical trial (NCT04543188), and they are specifically enrolling patients with BRAF V600-mutant cancers with and without brain metastasis [5]. However, rapid development of drug resistance was reported following BRAFi monotherapy, especially vemurafenib, despite the encouraging clinical benefits. This is potentially due to the emergence of BRAFV600E splicing variants which are insensitive to the BRAFi monotherapy, as well as the overactivation of wild-type BRAF after BRAFi inhibits mutated BRAFV600 [6].
Aiming to more effectively target MAPK pathways and reduce this resistance, BRAFi and its downstream MEKi were combined as a doublet treatment for BRAF-mutated melanomas. In the COMBI-d trial, trametinib was added to dabrafenib as a first-line systemic treatment of unresectable or metastatic BRAFV600E/K mutationpositive cutaneous melanoma. In comparison to treatment with dabrafenib monotherapy, treatment with both trametinib and dabrafenib was associated with prolonged median overall survival (OS) (25.1 months vs 18.7 months) and increased median Progression Free Survival (PFS) (11.0 months vs 8.8 months) [7]. The extended follow-up data from the COMBI-v trial demonstrated this combination continued to improve both PFS and OS (4-year PFS 21%; 4-year OS 37%) [8]. Additionally, in the coBRIM trial, the combination of vemurafenib and cobimetinib exhibited remarkably improved median OS (22.3 months vs 17.4 months) and PFS (12.3 months vs 7.2 months), compared to vemurafenib monotherapy after ≥5 years since the last patient was randomized [9]. Similarly, in the Columbus trial, encorafenib together with binimetinib led to a median PFS of 14.9 months and OS of 33.6 months, compared to a 9.6-month median PFS and a 23.5-month median OS with encorafenib monotherapy. Overall, in these clinical trials, treatment with BRAFi and MEKi all led to an objective response rate (ORR) over 60% and Complete Response Rate (CRR) above 10% [10].
Immunotherapy
The CTLA-4 and PD-1 blockade has been shown to improve antitumor T cell responses and tumor cell eradication in preclinical tumor models. The subsequent development of monoclonal antibodies against CTLA-4 (e.g. ipilimumab) and targeting PD-1 (e.g. pembrolizumab, nivolumab, atezolizumab) and the successful outcomes of ICIs in clinical trials with advanced melanoma patients has significantly transformed the melanoma treatment paradigm. In the CheckMate067 trial, untreated unresectable stage IIIIV patients were randomized into ipilimumab, nivolumab and nivolumab+ipilimumab treatment groups. With a follow-up of 6.5 years, the median OS was 19.9 months with ipilimumab, 36.9 months with nivolumab, and 72.1 months with nivolumab+ipilimumab. The median treatment-free interval was 1.9 months, 2.3 months, and 27.6 months with ipilimumab, nivolumab and nivolumab+ipilimumab respectively. In addition, after ipilimumab, nivolumab, and nivolumab+ipilimumab treatment, respectively 43%, 74%, and 81% of all the patients alive in follow-up were off treatment and never received subsequent systemic therapy [11- 13], indicating the remarkable success of combinational ICIs.
Triplet therapy
Based on the successful clinical outcomes of targeted therapy and immunotherapy, the combination of these effective modalities appears as a logical next step for BRAF-mutated melanoma treatment. In the IMspire150 clinical trial, unresectable stage III-IV patients were randomized into vemurafenib+cobimetinib+atezolizumab or vemurafenib+cobimetinib+placebo groups. With median follow up of 18.9 months, a remarkably prolonged median PFS (15.1 months) and duration of response (DOR) (21.0 months) were observed in the vemurafenib+cobimetinib+atezolizumab group, in comparison to the vemurafenib+cobimetinib+placebo group which demonstrated a median PFS of 10.6 months and DOR of 12.6 months [14]. In the KEYNOTE022 trial, previously untreated BRAFV600E/K-mutated advanced melanoma patients were randomly assigned into pembrolizumab+ dabrafenib+trametinib or dabrafenib+trametinib groups. With triplet and doublet treatment, respectively, the median PFS was 16.9 months vs 10.7 months; the PFS at 24 months was 41.0% vs 16.3%; the median DOR was 25.1 months vs 12.1 months; the OS at 24 months was 63.0% vs 51.7% [15]. All these results suggest clinically significant improvement in survival and duration of response with triplet treatment compared to with doublet treatment.
Sequence of systemic therapies for metastatic BRAF V600 mutant melanoma
While BRAFi/MEKi and ICIs have both been shown to result in significant clinical outcomes, a clinical dilemma has appeared regarding how to sequence these agents to exert the best outcomes in patients with BRAF mutated melanoma. Some studies suggest that while prior treatment with ICIs does not negatively impact patients’ responses to BRAFi, treatment with ICIs following BRAFi discontinuation leads to poor responses [16]. While BRAFi+MEKi demonstrates a better outcome than BRAFi singlet treatment and ICI doublet therapy leads to more success than ICI singlet therapy, it is of great interest to explore the sequence of BRAFi+MEKi therapy and ICI doublet therapy when combining them for treatment. Recently, the DREAMseq trial was designed to compare the effectiveness of the sequence of nivolumab+ipilimumab followed by dabrafenib+trametinib to the converse sequence (dabrafenib+trametinib followed by nivolumab+ipilimumab). The results show that the treatment sequence starting with nivolumab+ipilimumab results in improved OS (which became evident at 10 months) and long-term treatment-free survival [17]. This study has shed light on optimal sequencing of treatment with ICIs and BRAFi/MEKi; other questions including how to sequence PD-1 monotherapy and BRAFi/MEKi remain to be further explored.
Discussion
Therapies for advanced melanoma have rapidly evolved in the past decade, with substantial improvement in patient response and survival rate. ICIs, whose efficacy has been proven in various clinical trials such as CheckMate067, have become one of the preferred treatment modalities and induced the emergence of triplet treatment. In the recent clinical trials comparing triplet and doublet treatment, triplets have demonstrated even more promising outcomes. However, it is still noteworthy that, so far, all the reported clinical trials involving triplets have failed to include control groups of ICIs alone. Therefore, we are still unable to directly compare the efficacy of triplets and ICIs such as nivolumab+ipilimumab. Regarding safety, with the toxicities known to be mostly derived from targeted therapies, the severe adverse events and sum of toxicities remain to be a concern for triplet compared to doublet treatment for further clarification. The current clinical practice guideline in metastatic BRAF mutated melanoma is to start patients on dual immunotherapy with nivolumab + ipilimumab first if they are able to tolerate the potential toxicities.
Increasing understanding of molecular features in melanoma and tumor microenvironment has prompted the development of more potential treatments. For instance, the investigation of inhibitory co-receptors such as lymphocyte-activation gene-3 (LAG- 3) and T cell immunoglobulin mucin-3, as well as indeoleamine 2,3-di-oxygenase has led to RELATIVITY-047 trial. In this trial, relatlimab, the LAG-3 blocking antibody was combined with nivolumab and demonstrated a greater outcome than nivolumab treatment alone in untreated advanced melanoma [18]. Talimogene laherparepvec (T-VEC), an oncolytic herpes virotherapy expressing granulocyte macrophage colony-stimulating factor (GM-CSF), has been generated aiming to cause simultaneous tumor cell lysis and immune cell recruitment. With an ORR of 26% and CRR of 11% compared to an ORR of 6% and CRR of 1% with GM-CSF treatment, T-VEC has exhibited a higher efficacy in regressing tumors than immunostimulatory gene product alone. After demonstrating an improved durable response in patients with stage IIIB-IV melanoma, T-VEC was approved by the FDA in 2015 [19]. All these attractive approaches have provided us with more means of prolonging survival and enhancing the recovery rate in advanced melanoma patients [20].
With the development of a more intricate treatment landscape, the individualization of treatment for each patient has gradually attracted more attention with concerns that a “one size fits all” therapy might not be sufficient in treating every metastatic melanoma patient in the future. Optimizing the selection, sequence, and combination of different therapies, developing new effective therapies with substantial durable response, and balancing clinical benefit with adverse events in combinational therapies remain to be further explored in future studies.
Conclusion
The approval of targeted therapy and immunotherapy in the past decade has led to a revolution in the field of advanced melanoma treatment. Doublet and triplet therapies have further been designed for improved clinical outcome. Optimization of the selection and sequence of therapies still warrants further exploration in order to reach the maximal probability of inducing complete and durable remission and enhanced long-term outcomes for advanced melanoma patients.
Future Perspectives
For the successful treatment of advanced melanoma, paradigmchanging treatment modalities including the targeted therapy and immunotherapy have been developed in the past decade. Multiple clinical trials are currently undergoing that study and compare the efficacy of these therapies in different combinations and sequences. With these remarkable achievements, deeper understanding of advanced melanoma pathways and the mechanisms by which the drug resistance emerges remains necessary and will potentially lead to more effective therapies. Additionally, identification of new synthetic compounds targeting unique components in the molecular profile of advanced melanoma are still of great interest and expected to yield new treatment options. While the current immunotherapies that hamper the immune tolerance have shown great success, further exploration of the complex tumor microenvironment that supports the melanoma growth and promotes immune tolerance is expected to lead to the next breakthrough in immunotherapies for advanced melanoma.
Acknowledgement
High Throughput Screening Core at City of Hope is supported by the National Cancer Institute of the National Institutes of Health under award number P30CA033572. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of Interest
The authors declare no conflict of interest.
References
- Eroglu Z, Ribas A (2016) Combination therapy with BRAF and MEK inhibitors for melanoma: latest evidence and place in therapy. Ther Adv Med Oncol 8(1): 48-56.
- Cancer Genome Atlas Network (2015) Genomic classification of cutaneous melanoma. Cell 161(7): 1681-1696.
- Giugliano F, Crimini E, Tarantino P, Zagami P, Uliano J, et al. (2021) First line treatment of BRAF mutated advanced melanoma: Does one size fit all? Cancer Treat Rev 99:102253.
- Patel H, Yacoub N, Mishra R, White A, Long Y, et al. (2020) Current advances in the treatment of BRAF-mutant melanoma. Cancers (Basel) 12(2): 482.
- Vivek S, Martin G, Carey A, George A, Taofeek KO, et al. (2021) Trial in progress: Phase 1a/b study of PF-07284890 (brain-penetrant BRAF inhibitor) with/without binimetinib in patients with BRAF V600-mutant solid tumors. Journal of Clinical Oncology 39(15): TPS3152-TPS3152.
- Poulikakos PI, Persaud Y, Janakiraman M, Kong X, Ng C, et al. (2011) mRAF inhibitor resistance is mediated by dimerization of aberrantly spliced BRAF(V600E). Nature 480(7377): 387-390.
- Curti BD, Faries MB (2021) Recent advances in the treatment of melanoma. N Engl J Med 384(23): 2229-2240.
- Robert C, Grob JJ, Stroyakovskiy D, Karaszewska B, Hauschild A, et al. (2019) Five-year outcomes with dabrafenib plus trametinib in metastatic melanoma. N Engl J Med 381(7): 626-636.
- Ascierto PA, McArthur GA, Dréno B, Atkinson V, Liszkay G, et al. (2016) Cobimetinib combined with vemurafenib in advanced BRAF(V600)-mutant melanoma (coBRIM): updated efficacy results from a randomised, double-blind, phase 3 trial. Lancet Oncol 17(9): 1248-1260.
- Ascierto PA, Dummer R, Gogas HJ, Flaherty KT, Arance A, et al. (2020) Update on tolerability and overall survival in COLUMBUS: landmark analysis of a randomised phase 3 trial of encorafenib plus binimetinib vs vemurafenib or encorafenib in patients with BRAF V600-mutant melanoma. Eur J Cancer 126: 33-44.
- Wolchok JD, Vanna Chiarion, Gonzalez R, Jacques Grob, Rutkowski P, et al. (2021) CheckMate 067: 6.5-year outcomes in patients with advanced melanoma. Journal of Clinical Oncology 39(15): 9506-9506.
- Eggermont AM, Chiarion-Sileni V, Grob JJ, Dummer R, Wolchok JD, et al. Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N Engl J Med 375(19): 1845-1855.
- Tarhini AA, Lee SJ, Hodi FS, Rao UNM, Cohen GI, et al. (2020) Phase III study of adjuvant ipilimumab (3 or 10mg/kg) versus high-dose interferon Alfa-2b for resected high-risk melanoma: North American Intergroup E1609. J Clin Oncol 38(6): 567-575.
- McArthur G, Stroyakovskiy D, Gogas H, Robert C, Lewis K, et al. (2020) Abstract CT012: Evaluation of atezolizumab (A), cobimetinib (C), and vemurafenib (V) in previously untreated patients with BRAFV600 mutation-positive advanced melanoma: Primary results from the phase 3 IMspire150 trial. Cancer Res 80(16): CT012-CT012.
- Ferrucci PF, Di Giacomo AM, Del Vecchio M, Atkinson V, Schmidt H, et al. (2020) KEYNOTE-022 part 3: a randomized, double-blind, phase 2 study of pembrolizumab, dabrafenib, and trametinib in BRAF-mutant melanoma. J Immunother Cancer 8(2): e001806.
- Ackerman A, Klein O, McDermott DF, Wang W, Ibrahim N, et al. (2014) Outcomes of patients with metastatic melanoma treated with immunotherapy prior to or after BRAF inhibitors. Cancer 120(11): 1695-1701.
- Atkins MB, Lee SJ, Chmielowski B, Ribas A, Tarhini AA, et al. DREAMseq (Doublet, Randomized Evaluation in Advanced Melanoma Sequencing): A phase III trial-ECOG-ACRIN EA6134. Journal of Clinical Oncology 39(36): 356154-356154.
- Tawbi, HA, Schadendorf D, Lipson EJ, Ascierto PA, Matamala L, et al. (2022) Relatlimab and Nivolumab versus Nivolumab in untreated advanced melanoma. New England Journal of Medicine 386(1): 24-34.
- RobertHI, Collichio FA, Amatruda T, Senzer NN, Chesney J, et al. (2013) OPTiM: A randomized phase III trial of talimogene laherparepvec (T-VEC) versus subcutaneous (SC) granulocyte-macrophage colony-stimulating factor (GM-CSF) for the treatment (tx) of unresected stage IIIB/C and IV melanoma. Journal of Clinical Oncology 31(8): LBA9008-LBA9008.
- Hepner A, Salgues A, Anjos CAD, Sahade M, Camargo VP, et al. (2017) Treatment of advanced melanoma - A changing landscape. Rev Assoc Med Bras 63(9): 814-823.
© 2022. Yan Xing. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






