- Submissions

Full Text
Novel Approaches in Cancer Study
CAR-T Cell, a Therapeutic Tune-up for Colorectal Cancer Treatment
Rachel M Morris, Shayna N Scott, Toni O Mortimer and Kim L O’Neill*
Department of Microbiology and Molecular Biology, USA
*Corresponding author: Kim L O’Neill, Department of Microbiology and Molecular Biology, Provo, Utah 84602, USA
Submission: January 28, 2022 Published: February 10, 2022

ISSN:2637-773XVolume6 Issue5
Abstract
Colorectal Cancer (CRC) is a leading cause of cancer-related deaths. Prevention and screening have allowed CRC rates to plateau in several countries; however, CRC continues to be challenging to treat and often leaves patients with chronic pain. Conventional treatments, such as chemotherapy and radiation, often result in resistant disease further complicating treatment. Consequentially, interest in novel immunotherapeutic treatments has grown substantially within the past few decades to provide additional treatment options. Chimeric Antigen Receptor (CAR)-T cells are an especially attractive strategy to add to the immunotherapy toolbox because of the successful treatment of hematological malignancies. However, CAR-T cell therapy faces several additional challenges when applied to treating solid tumors, including CRC. Modifying the CAR-T cell design and understanding obstacles associated with the Tumor Microenvironment (TME) may present ways to improve current CAR-T cell treatments for CRC. In this mini-review, we discuss the evolution of CAR-T cells, molecular biology of the TME, target selection, and CRC targets in preclinical and clinical trial stages.
Keywords: CAR-T cell therapy; Checkpoint inhibitors; Colorectal cancer; Tumor microenvironment
Introduction
Colorectal Cancer (CRC) includes malignant neoplasms of the colon, rectum, and anus. In the USA, CRC ranks third in the number of total cancers diagnosed among both men and women, and second in cancer mortality. CRC incidence and mortality is rapidly increasing in Asia, Eastern Europe, and developing countries in South America. CRC rates in high Human Development Index (HDI) countries, such as Australia, New Zealand, some Western European countries, and the USA are about 4-fold higher than less developed countries; however, many of these countries’ case rates have begun to stabilize or even decline possibly due to increased prevention and early detection efforts [1,2]. It is estimated that approximately 95% of colorectal cancers originate from adenomatous polyps, which are benign neoplasms that are often characterized as having potential malignancy and dysplastic epithelium [3]. Therefore, screening programs and colonoscopy are especially important to be employed for early detection and removal of polyps. CRC is also highly associated with poor diet consisting of high processed meat and low whole grain intake and obesity [4]. Current treatments for CRC include surgery, neoadjuvant radiotherapy, chemotherapy, and targeted therapy. The conventional treatment for advanced CRC consists of 5-fluouracil (5-FU) and leucovorin in combination with irinotecan or oxaliplatin. However, patients with metastatic disease often become resistant to 5-FU [5]. In recent years, monoclonal antibodies bevacizumab and cetuximab became available for CRC treatment [5]. Despite these novel treatments, the survival time for metastatic CRC patients is only about thirty months [6]. Also, over 70% of CRC patients report having pain, and those that survive longer may suffer from chronic pain [7]. Therefore, CRC is a highly debilitating disease that is in urgent need of additional treatment options. Immunotherapies are currently being developed to treat CRC, especially CAR-T cell therapy. In this mini-review, we will discuss CAR-T cell development, challenges, potential CRC-specific targets, and the current status of these targets in clinical trials.
The Evolution of CAR-T Cells
CAR-T cells contain a genetically engineered non-Major Histocompatibility (MHC)-restricted receptor incorporated with a single-chain Variable Fragment (scFv) of an antibody. This receptor is engineered to be antigen specific, thus allowing CAR-T cells to be potentially used to target specific antigens on tumor cells [8]. Over time, CAR-T cells have undergone four major stages of design. The first-generation design contains a T-cell receptor CD3-ζ domain, which initiates T cell receptor (TCR) signaling [9]. However, the first generation lacked the ability to persist and proliferate effectively in vivo [10,11].
Two signals are needed for T cell activation and proliferation: one signal from the CD3-ζ domain, and another signal from a costimulatory domain (most often either CD28 or 4-1BB) [8]. The following generations of CAR-T cells are distinguished by the presence of one or more of these distinct costimulatory domains. The second generation, also termed the next generation, possesses one of these costimulatory domains, which enhances T cell persistence, proliferation, and cytotoxicity. As a member of the immunoglobulin superfamily, CD28’s principal function is to increase TCR signaling [12]. This increase in signaling lowers the TCR binding threshold required for effective T cell activation. Costimulation from CD28 also leads to clonal proliferation, differentiation, survival, and increased cytokine production [12]. In addition to CD28, there are also other molecules that influence T cell activation. As a member of the Tumor Necrosis Factor (TNF) receptor superfamily, 4-1BB improves cytokine secretion and T cell proliferation [12]. 4-1BB also diminishes T cells’ ability to be suppressed by transforming growth factor β (TGF-β) suppression, a cytokine that inhibits TCR signaling [12,13]. However, it should be noted that the functions of these costimulatory receptors in the context of CARs are not as well understood as they are in other clinical settings [12]. Research has shown that CARs stimulated with either CD28 or 4-1BB lead to enhanced cytokine secretion; however, there are differences in effects between these two costimulatory domains [14-17]. Recent studies have shown that CARs with CD28 domains promote faster T cell activation, which leads to increased antitumor activity and a faster rapid tumor clearance when compared to CARs with 4-1BB domains [12,14-18]. However, CARs engineered with 4-1BB domains demonstrated higher persistence, longer lasting responses, and decreased risk for harmful side effects than CARs with CD28 domains [12,14-19].
With the variations in T cell activity, as well as the different signaling pathways utilized by the two costimulatory domains, researchers have wondered if combining the two costimulatory domains into one CAR would result in increased T cell activity, proliferation, and persistence. Previous research has shown that costimulation of T cells with both domains results in increased intracellular signaling and increased antitumor efficacy when compared to second-generation CARs [20,21]. This leads to the third generation of CAR-T cell, which was engineered to possess two costimulatory domains [22]. While the combination of costimulatory domains can increase the efficacy and persistence of CAR-T cells [20-24], it may also result in increased T cell exhaustion and cytokine release syndrome (CRS) risk [11,25]. CRS is a systemic inflammatory response that is often triggered by massive stimulation of T cells and other immune cells, resulting in a mass release of cytokines [25,26]. Symptoms of CRS range from mild symptoms such as fatigue, headaches, and rashes, to more severe symptoms such as hypotension, organ failure, and death [25]. The mechanism as to how CAR-T cells stimulate CRS is not fully understood and, therefore, further study is required to understand the role of costimulatory domains on CRS severity [25].
To combat these immunological consequences, a fourth generation, known as T cells redirected for antigen-unrestricted cytokine-initiated killing (TRUCKs), was engineered. Currently, TRUCKs are CAR-T cells that possess the same antitumor attack mechanisms of the previous generations, but are engineered with a third stimulatory signal, which should increase proliferation and efficacy of the CAR-T cells [27,28]. In addition to the functions that previous generations possess, TRUCKs release both transgenic cytokines and immune stimulatory cytokines [27-29]. These cytokines can lead to activation of the innate immune system, prevention of TGF-β repression, increased persistence and recruitment of other immune cells to target the cancer cells [27,29]. Because TRUCKs are a recent development, little clinical data exist on them. Some TRUCKs have been engineered with an iCasp9 suicide switch that should induce apoptosis in the TRUCKs in order to evade toxicity problems [30]. Other clinical studies have also demonstrated that fourth generation CAR-T cells can result in reduced CRS, but further studies are needed to fully understand the efficiency and safety of this fourth generation of CAR-T cells [31-33]. While progress has been made in understanding and improving on the mechanisms of CAR-T cell therapy, further clinical research is needed to better understand the efficacy and safety of the therapy in general.
Major Challenges in CAR-T Cell Therapy
A tumor is more than an accumulation of cancer cells and its micro environment is a challenge that must be considered for effective delivery of CAR-T cell therapy. The Tumor Microenvironment (TME) consists of a myriad of cancer cells, secreted chemokines and cytokines, signaling molecules, extracellular matrix components, and infiltrating immune cells [34,35]. Apart from being a site for local and systemic inflammation, the TME is also largely involved in tumor progression, drug resistance, and immune cell recruitment and activation [34,35]. Immunosuppression is, in part, achieved by the TME through the secretion of chemokines and cytokines by tumor-associated macrophages, fibroblasts, regulatory T cells (Tregs), and tumor cells [36,37]. Tregs may also express coinhibitory molecules, including CTLA-4, PD-1, TIGIT, TIM-3, and LAG-3 [37]. TNF-α is one cytokine essential for signaling immune cells and trafficking CAR-T cells to the TME. It is mediated by two receptors, pro-apoptotic TNF-R1 and anti-apoptotic TNF-R2 [38]. TNF-R2 is expressed by some immune cells, like Tregs, as well as some cancer cells and mediates tumor resistance to TNF-α [39]. As a tumor forms, rapidly proliferating cancer cells produce inhibitory metabolites, increase their nutrient uptake, and create an acidic environment through the production of lactic acid and carbonic acid during aerobic glycolysis and oxidative phosphorylation [37,40]. A metabolically hostile TME caused by nutrient depletion, hypoxia, and acidity makes it difficult for immune cells and CAR-T cells to survive [36,40,41]. This metabolic phenotype is in part caused by the Warburg effect, or metabolic reprogramming essential for supporting cancer cell proliferation and progression of malignancies [42,43].
In addition to the unfavorable molecular characteristics of the TME, there are also physical barriers that affect the efficacy of CAR-T therapy [44]. These barriers consist of an extracellular matrix and surrounding stroma [45]. Tumor myeloid cells and fibroblasts also influence the development of an extracellular matrix that impedes the penetration of CAR-T cells [46]. These challenges presented by the physical barriers of tumors, as well as a hostile microenvironment, impact the therapeutic possibilities of CAR-T cell treatment for solid tumors and may influence the persistence of CAR-T cells [47]. Sustained persistence of CAR-T cells is crucial for long-term remission of patients.
To overcome some of these challenges when treating solid tumors, combination therapies are emerging to improve poor clinical outcomes for patients with metastatic CRC. For example, introducing Immune Checkpoint Inhibitors (ICIs) that block signaling pathways in charge of negative regulation of the immune system may help inhibit cytotoxic activity. Clinical trial has shown promising results in the treatment of solid tumors [48]. ICIs in conjunction with CAR-T cell therapy may also help combat poor CAR-T cell persistence within the tumor mass. Various ICIs (anti- PD-1; anti-PD-L1; anti-CTLA-4) have been effective for patients with CRC. Nivolumab, pembrolizumab, atezolizumab, and ipilimumab are examples of checkpoint inhibitors that are FDA approved and have shown encouraging results in the treatment of various solid tumors [48]. Adoptive cellular immunotherapies may also be promising in the treatment of solid tumors depending on the target. One target under clinical evaluation is cancer embryonic antigen (CEA) (Table 1). The clinical importance of this antigen will be discussed later in this review. CEA is involved in cellular adhesion and is a sensitive tumor biomarker, which is often increased in CRC tissues and serum [49]. In a study performed by Fan et al. [50], a construct containing a second-generation CEA-targeting CAR was used to overexpress B-cell lymphoma-extra-large (BclxL). Bcl-xL, when activated, influences T cell survival and function. By overexpressing exogenous Bcl-xL in vivo in an anti-CEA CAR-T, CEA-expressing tumor cells were destroyed, and CAR-T persistence increased. Thus, this study also suggests the potential for improved CAR-T cell immunotherapy for solid tumors through exogenous expression of persistent genes.
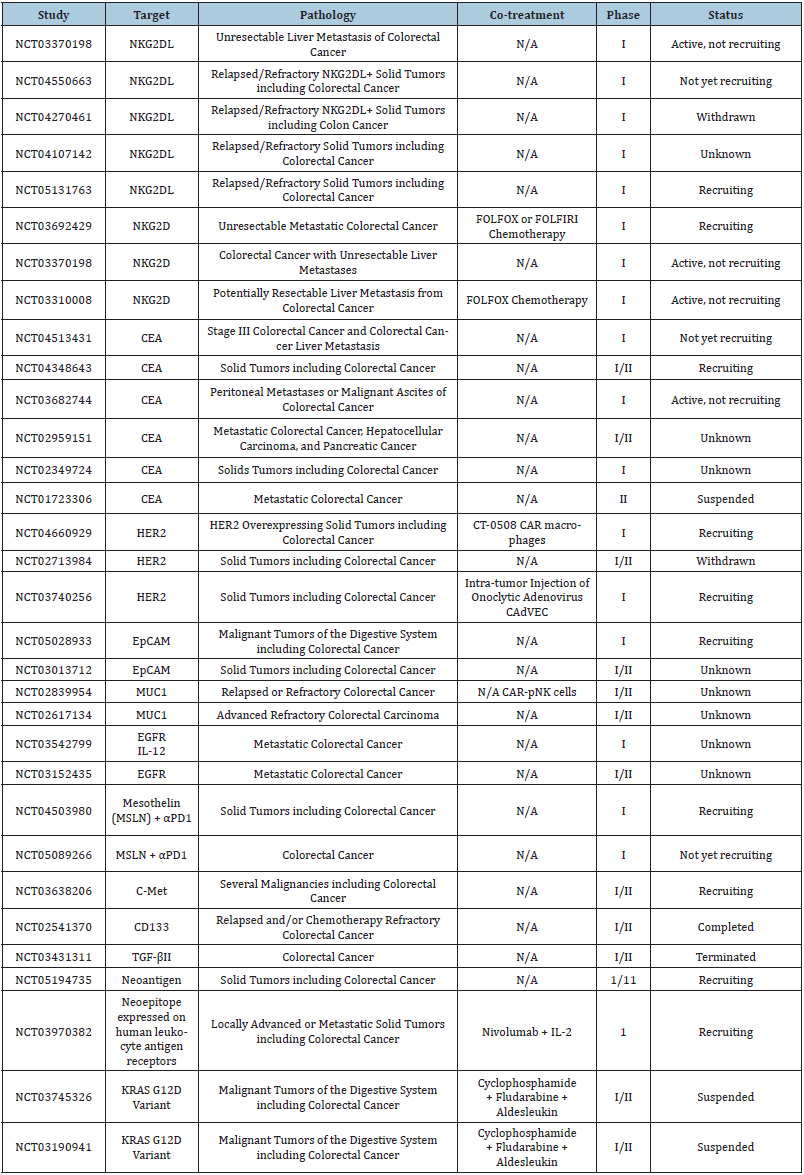
Table 1: A list of all clinical trials (as of January 2022) related to treating colon cancer with CAR-T cells.
Tricky Target Selection, Off-Target Toxicity, & Antigen Escape
In addition to overcoming obstacles of the TME, target selection remains an important and fundamental CAR-T cell design feature that cannot be overlooked. However, finding the “ideal target” often is its own challenge. Generally, the best CAR-T therapies target an antigen that provides high levels of specificity and coverage. High specificity and coverage provide patients with a safe and efficacious treatment, which is required to improve CAR-T cell therapy targeting solid tumors [51]. Specificity and coverage also address two major limitations of CAR-T therapy target selection:
a. severe toxicity from CAR-T cells attacking healthy normal tissues, also termed on-target/off-target toxicity, and
b. antigen escape/loss due to inadequate targeting, which may lead to relapse of disease [52].
Target selection must be closely evaluated to decide which antigen is best for a patient’s cancer subtype. Antigens targeted by CAR-T cells may include a wide range of proteins or carbohydrates. CAR-T cell function is dependent on antibody fragments, which support the redirection of T cell specificity against tumor antigens. To prevent adverse toxic effects, the ideal antigen is a Tumor- Specific Antigen (TSA) that is uniquely expressed on tumor cells and not displayed on healthy tissues. However, little is known about TSAs, making them difficult to identify and target. Also, identifying each patient’s TSA and then engineering personalized CAR-T cells is costly and, therefore, not a practical option for most patients [36]. Although a TSA would provide the ideal target, many antigens that undergo CAR-T targeting are not TSAs but tumor-associated antigens (TAA), which are generally highly expressed by tumors and simultaneously expressed by healthy tissues, but at lower levels. This makes target selection easier at the cost of increasing risk for on-target/off-target toxicity [53]. In addition, antigens may vary between tumor subtypes, indicating a need for screening prior to treatment to ensure expression of the target [54].
Although tumors may be responsive to targeted therapy during initial treatment, malignant cells may later present partial or complete loss of antigen resulting in tumor resistance against the initial CAR construct and lack of coverage [55]. Interestingly, despite higher success with CAR-T therapies for hematological malignancies, antigen loss is still observed in clinical trials targeting CD19 for acute lymphoblastic leukemia (ALL), primary mediastinal large B cell lymphoma (PMLBCL), and chronic lymphocytic leukemia (CLL) [56-59]. Thus, current research suggests a general need for new strategies to prevent antigen escape in all CAR-T therapies. One strategy includes generating T cells that have dual-targeting abilities. Dual-targeted CAR-T cells usually have two unique CAR molecules with different binding domains, or two binding domains that are in tandem on the one CAR molecule to make a TanCAR. Regardless of CAR molecule design, either antigen can elicit immune activity to provide higher coverage, prevent the escape of one antigen, and avoid antigen loss relapse. However, several concerns related to this strategy include increased potential risk of CRS due to increased cytokine release from CAR-T cell expansion and possible escape of two antigens [52]. In one case study, two different CAR groups targeting EGFR and CD133 were sequentially infused to treat cholangiocarcinoma (CCA). Treatment resulted in a partial response from both therapies, but infusion-related toxicities were observed [60]. While the dual-targeted CAR-T cell approach is an attractive strategy, further investigation will be required prior to optimization.
Targets in Preclinical & Clinical Trial Stages
There are several biomarkers that are currently undergoing testing to be used as potential targets for CAR-T cell therapy in CRC treatment. One is doublecortin-like kinase 1 (DCLK1), which is displayed on tumor stem cells residing in the mouse gut. Interestingly, this biomarker is not detected on normal stem cells when performing lineage-tracing experiments [61]. In humans, DCLK1 levels may be related to elevated risk for metastasis, recurrence, and morbidity due to CRC [62]. Recently, CAR-T cells targeting DCLK1 were tested in mice with CRC, reducing tumor growth by about 50 percent. Interestingly, the CAR-T cells also showed increased IFN-γ release when exposed to CRC cells growing three-dimensionally, as compared to IFN-γ levels when CARs were exposed to CRC in two-dimensional growth [63]. These studies suggest that DCLK1-positive tumor stem cells may be a potential immunotherapy target. Another biomarker being tested as a potential CAR-T cell target for treating CRC is guanylyl cyclase C (GUCY2C), which is a membrane-bound receptor that promotes cyclic GMP activation to further activate cGMP-dependent protein kinase II (PKGII) and promote downstream activation via phosphorylation. Results include electrolyte and water secretion and tumor suppressor increase [64]. Magee et al. [65] tested human GUCY2C-targeted CAR-T cells in mice. Results indicated that GUCY2C murine CAR-T cells successfully killed human CRC cells displaying GUCY2C from a human xenograft in immunodeficient mice. The study also revealed that the GUCY2C CAR-T cells protected the syngeneic mice from murine CRC cells expressing human GUCY2C [65,66]. These studies present encouraging findings of novel targets that may be tested in future clinical trials.
NKG2D is a regulatory receptor expressed by natural killer (NK) cells, some CD4+ T cells, CD8+ T cells, and some γδ T cells. The receptor is known to regulate activation of innate and adaptive immune functions and is a major activating receptor on NK cells [67]. There are 8 NKG2D ligands (NKG2DLs), including six different long 16 (UL16)-binding proteins (ULBP1-6) and MHC class I chain-related proteins A and B (MICA and MICB) that interact with NKG2D [66]. NKG2D is not displayed or is at only minimal levels on healthy tissues; however, elevated expression may be induced by cell transformation and DNA damage [68]. Deng et al. [69] transduced a non-viral third generation NKG2D CAR construct into T cells. Results indicated that the NKG2D CAR-T cells were cytotoxic against human CRC in a dose-dependent manner and secreted significantly higher levels of IFN-γ and IL-2 than untransduced T cells. Treatment significantly extended overall survival of xenograft mouse models inoculated with HCT-116 cells. CAR-NK cells are undergoing investigation to be used to target NKG2D ligands. CARNK cells have the advantage of less CRS risk compared to CAR-T cells [70]. However, more testing is required to determine if CARNK cells will be used in future treatments.
Carcinoembryonic Antigen (CEA) is an acid glycoprotein and CRC biomarker [71,72]. Cha et al. [73] showed that anti-CEA CAR-T cells combined with an anti-CEA-IL2 immunocytokine administered every 3 days could eliminate MC38/CEA tumor growth. Mice that were re-challenged with tumor demonstrated immunity. In a phase I clinical trial, CAR-T cells were administered to CEA-positive CRC patients. 7 of the 10 patients became stable after treatment, while 2 showed tumor regression and another 2 were stable for over 30 weeks. However, fever, duodenum perforation, and lymphocyte count decrease were observed post-cell therapy [74].
Conclusion
As CAR-T cell therapy continues to develop, new strategies to overcome TME, toxicity, and the search for novel biomarkers as continue. We have provided a brief overview of the current innovations in CAR-T cell therapy related to CRC and highlighted a few of the biomarkers under preclinical and clinical investigation. As CAR-T cell therapy and target selection become more sophisticated, immunotherapy may become the new standard in caring for patients with CRC. The potential for CAR-T cell therapy targeting CRC remains an important research area for enhancing both the CAR-T strategies and CRC treatment.
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, et al. (2020) Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3): 209-249.
- Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, et al. (2017) Global patterns and trends in colorectal cancer incidence and mortality. Gut 66(4): 683-691.
- Aarons CB, Shanmugan S, Bleier JIS (2014) Management of malignant colon polyps: Current status and controversies. World J Gastroenterol 20(43): 16178.
- Cheng Y, Ling Z, Li L (2020) The intestinal microbiota and colorectal cancer. Front Immunol 11:
- Van Der Jeught K, Xu HC, Li YJ, Bin LX, Ji, G (2018) Drug resistance and new therapies in colorectal cancer. World J Gastroenterol 24(34): 3834-3848.
- Kishore C, Bhadra P (2020) Current advancements and future perspectives of immunotherapy in colorectal cancer research. Eur J Pharmacol 893: 173819.
- Zielińska A, Włodarczyk M, Makaro A, Sałaga M, Fichna J (2021) Management of pain in colorectal cancer patients. Crit Rev Oncol Hematol 157: 103122.
- Petty AJ, Heyman B, Yang Y (2020) Chimeric antigen receptor cell therapy: Overcoming obstacles to battle cancer. Cancers (Basel) 12(4): 842.
- Brudno JN, Kochenderfer JN (2017) Chimeric antigen receptor T-cell therapies for lymphoma. Nat Rev Clin Oncol 15(1): 31-46.
- Pule MA, Savoldo B, Myers GD, Rossig C, Russell HV, et al. (2008) Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med 14(11): 1264-1270.
- Huang R, Li X, He Y, Zhu W, Gao L, et al. (2020) Recent advances in CAR-T cell engineering. J Hematol Oncol 13(1): 86.
- Van Der Stegen SJC, Hamieh M, Sadelain M (2015) The pharmacology of second-generation chimeric antigen receptors. Nat Rev Drug Discov 14(7): 499-509.
- Oh SA, Li MO (2013) TGF-β: Guardian of T Cell Function. J Immunol 191(8): 3973-3979.
- Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, et al. (2017) Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med 377(26): 2531-2544.
- Kawalekar OU, O’Connor RS, Fraiett JA, Guo L, McGettigan SE, et al. (2016) Distinct signaling of coreceptors regulates specific metabolism pathways and impacts memory development in CAR T cells. Immunity 44(2): 380-390.
- Zhao Z, Condomines M, van der Stegen SJC, Perna F, Kloss CC, et al. (2015) Structural design of engineered co-stimulation determines tumor rejection kinetics and persistence of CAR T cells. Cancer Cell 28(4): 415-428.
- Ying Z, He T, Wang X, Zheng W, Lin N, et al. (2019) Parallel comparison of 4-1BB or CD28- co-stimulated CD19-targeted CAR-T cells for B cell non-hodgkin’s lymphoma. Mol Ther Oncolytics 15: 60-68.
- Salter AI, Ivey RG, Kennedy JJ, Voillet V, Rajan A, et al. (2018) Phosphoproteomic analysis of chimeric antigen receptor signaling reveals kinetic and quantitative differences that affect cell function. Sci Signal 11(544): eaat6753.
- Long AH, Haso WM, Shern JF, Wanhainen KM, Murgai M, et al. (2015) 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat Med 21(6): 581-590.
- Carpenito C, Milone MC, Hassan R, Simonet JC, Lakhal M, et al. (2009) Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc Natl Acad Sci 106(9): 3360-3365.
- Wang J, Jensen M, Lin Y, Sui X, Chen E, et al. (2007) Optimizing adoptive polyclonal T cell immunotherapy of lymphomas, using a chimeric T cell receptor possessing CD28 and CD137 costimulatory domains. Hum Gene Ther 18(8): 712-725.
- Schmidts A, Maus MV (2018) Making CAR T cells a solid option for solid tumors. Front Immunol 9: 2593.
- Cheng J, Zhao L, Zhang Y, Qin Y, Guan Y, et al. (2019) Understanding the mechanisms of resistance to CAR T-cell therapy in malignancies. Front Oncol 9: 1237.
- Enblad G, Karlsson H, Gammelgård G, Wenthe J, Lövgren T, et al. (2018) A phase I/IIa trial using CD19-targeted third-generation CAR T cells for lymphoma and leukemia. Clin Cancer Res 24(24): 6185-6194.
- Shimabukuro-Vornhagen A, Gödel P, Subklewe M, Stemmler HJ, Schlößer HA, et al. (2018) Cytokine release syndrome. J Immunother Cancer 6(1): 56.
- Freyer CW, Porter DL (2020) Cytokine release syndrome and neurotoxicity following CAR T-cell therapy for hematologic malignancies. J Allergy Clin Immunol 146(5): 940-948.
- Petersen CT, Krenciute G (2019) Next generation CAR T cells for the immunotherapy of high-grade glioma. Front Oncol 9: 69.
- Li D, Li X, Zhou WL, Huang Y, Liang X, et al. (2019) Genetically engineered T cells for cancer immunotherapy. Signal Transduct Target Ther 41(4): 1-17.
- Chmielewski M, Abken H (2020) TRUCKS, the fourth-generation CAR T cells: Current developments and clinical translation. Adv. Cell Gene Ther 3(3): e84.
- Zhou X, Tu S, Wang C, Huang R, Deng L, et al. (2020) Phase I trial of fourth-generation anti-CD19 chimeric antigen receptor T cells against relapsed or refractory B cell non-hodgkin lymphomas. Front Immunol 11: 564099.
- Chang LJ, Dong L, Liu YC, Tsao ST, Li YC, et al. (2016) Safety and efficacy evaluation of 4SCAR19 chimeric antigen receptor-modified T cells targeting b cell acute lymphoblastic leukemia - three-year follow-up of a multicenter phase I/II study. Blood 128(22): 587-587.
- Zhang JP, Zhang R, Tsao ST, Liu YC, Chen X, et al. (2018) Sequential allogeneic and autologous CAR-T-cell therapy to treat an immune-compromised leukemic patient. Blood Adv 2(14): 1691-1695.
- Luangwattananun P, Junking M, Sujjitjoon J, Wutti-in Y, Poungvarin N, et al. (2021) Fourth-generation chimeric antigen receptor T cells targeting folate receptor alpha antigen expressed on breast cancer cells for adoptive T cell therapy. Breast Cancer Res Treat 186(1): 25-36.
- Kasprzak A (2021) The role of tumor microenvironment cells in Colorectal Cancer (CRC) Cachexia. Int J Mol Sci 22(4): 1565.
- Wang J, Wang J, Gu Q, Yang Y, Ma Y, et al. (2021) TGFβ1: An indicator for tumor immune microenvironment of colon cancer from a comprehensive analysis of TCGA. Front Genet 12: 612011.
- Liu B, Yan L, Zhou M (2019) Target selection of CAR T cell therapy in accordance with the TME for solid tumors. Am J Cancer Res 9(2): 228.
- Rao D, Verburg F, Renner K, Peeper DS, Lacroix R, et al. (2021) Metabolic profiles of regulatory T cells in the tumour microenvironment. Cancer Immunol Immunother 70(9): 2417-2427.
- Cabal-Hierro L, Lazo PS (2012) Signal transduction by tumor necrosis factor receptors. Cell Signal 24(6): 1297-1305.
- Josephs SF, Ichim TE, Prince SM, Kesari S, Marincola FM, et al. (2018) Unleashing endogenous TNF-alpha as a cancer immunotherapeutic. J Transl Med 16(1): 242.
- Nachef M, Ali AK, Almutairi SM, Lee SH (2021) Targeting SLC1A5 and SLC3A2/SLC7A5 as a potential strategy to strengthen anti-tumor immunity in the tumor microenvironment. Front Immunol 12: 624324.
- Reinfeld B, Madden M, Wolf M, Chytil A, Bader JE, et al. (2021) Cell programmed nutrient partitioning in the tumor microenvironment. Nature 593(7858): 282-288.
- Icard P, Shulman S, Farhat D, Steyaert JM, Alifano M, et al. (2018) How the Warburg effect supports aggressiveness and drug resistance of cancer cells? Drug Resist Updat 38: 1-11.
- Vaupel P, Schmidberger H, Mayer A (2019) The Warburg effect: essential part of metabolic reprogramming and central contributor to cancer progression. Int J Radiat Biol 95(7): 912-919.
- Gallo G, Vescio G, Paola G, Sammarco G (2021) Therapeutic targets and tumor microenvironment in colorectal cancer. J Clin Med 10(11): 2295.
- Tang XY, Ding YS, Zhou T, Wang X, Yang Y (2021) Tumor-tagging by oncolytic viruses: A novel strategy for CAR-T therapy against solid tumors. Cancer Lett 503: 69-74.
- Xia AL, Wang XC, Lu YJ, Lu XJ, Sun B (2017) Chimeric-Antigen Receptor T (CAR-T) cell therapy for solid tumors: challenges and opportunities. Oncotarget 8(52): 90521-90531.
- Scarfò I, Maus MV (2017) Current approaches to increase CAR T cell potency in solid tumors: Targeting the tumor microenvironment. J Immunother Cancer 5: 28.
- Dai Y, Zhao W, Yue L, Dai X, Rong D, et al. (2021) Perspectives on immunotherapy of metastatic colorectal cancer. Front Oncol 11: 2113.
- Li H, Yang C, Cheng H, Huang S, Zheng Y (2021) CAR-T cells for colorectal cancer: Target-selection and strategies for improved activity and safety. J Cancer 12(6): 1804-1814.
- Fan J, Das JK, Xiong X, Chen H, Song J (2021) Development of CAR-T cell persistence in adoptive immunotherapy of solid tumors. Front Oncol 10: 574860.
- Wei J, Han X, Bo J, Han W (2019) Target selection for CAR-T therapy. J Hematol Oncol 12(1): 62.
- Wang Z, Wu Z, Liu Y, Han W (2017) New development in CAR-T cell therapy. J Hematol Oncol 101(1): 53.
- Janelle V, Rulleau C, Del Testa S, Carli C, Delisle JS (2020) T-cell immunotherapies targeting histocompatibility and tumor antigens in hematological malignancies. Front Immunol 11: 276.
- Edeline J, Houot R, Marabelle A, Alcantara M (2021) CAR-T cells and BiTEs in solid tumors: challenges and perspectives. J Hematol Oncol 14(1): 65.
- Sterner RC, Sterner RM (2021) CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J 114(11): 1-11.
- Maude SL, Teachey DT, Rheingold SR, Shaw PA, Aplenc R, et al. (2016) Sustained remissions with CD19-specific chimeric antigen receptor (CAR)-modified T cells in children with relapsed/refractory ALL. Journal of Clinical Oncology 34(15): 3011-3011.
- Park JH, Riviere I, Wang X, Bernal Y, Purdon T, et al. (2015) Efficacy and safety of CD19-targeted 19-28z CAR modified T cells in adult patients with relapsed or refractory B-ALL. Journal of Clinical Oncology 33(15): 7010.
- Yu H, Sotillo E, Harrington C, Wertheim G, Paessler M, et al. (2017) Repeated loss of target surface antigen after immunotherapy in primary mediastinal large B cell lymphoma. Am J Hematol 92(1): E11-E13.
- Evans AG, Rothberg PG, Burack WR, Huntington SF, Porter DL, et al. (2015) Evolution to plasmablastic lymphoma evades CD19-directed chimeric antigen receptor T cells. Br J Haematol 171(2): 205-209.
- Feng K, Guo Y, Liu Y, Dai H, Wang Y, et al. (2017) Cocktail treatment with EGFR-specific and CD133-specific chimeric antigen receptor-modified T cells in a patient with advanced cholangiocarcinoma. J Hematol Oncol 10(1): 4.
- Nakanishi Y, Seno H, Fukuoka A, Ueo T, Yamaga Y, et al. (2012) Dclk1 distinguishes between tumor and normal stem cells in the intestine. Nat Genet 45(1): 98-103.
- Weygant N, Ge Y, Qu D, Kaddis JS, Berry WL, et al. (2016) Survival of patients with gastrointestinal cancers can be predicted by a surrogate microRNA signature for cancer stem-like cells marked by DCLK1 kinase. Cancer Res 76(14): 4090-4099.
- Sureban SM, Berahovich R, Zhou H, Xu S, Wu L, et al. (2020) DCLK1 Monoclonal antibody-based CAR-T cells as a novel treatment strategy against human colorectal cancers. Cancers (Basel) 12(1): 54.
- Aka AA, Rappaport JA, Pattison AM, Sato T, Snook AE, et al. (2017) Guanylate cyclase c as a target for prevention, detection, and therapy in colorectal cancer. Expert Rev Clin Pharmacol 10(5): 549-557.
- Magee MS, Abraham TS, Baybutt TR, Flickinger JC, Ridge NA, et al. (2018) Human GUCY2C-targeted chimeric antigen receptor (CAR)-expressing T cells eliminate colorectal cancer metastases. Cancer Immunol Res 6(5): 509-516.
- Jin KT, Chen B, Liu YY, Lan Huan R, Yan JP (2021) Monoclonal antibodies and chimeric antigen receptor (CAR) T cells in the treatment of colorectal cancer. Cancer Cell Int 21(1): 83.
- Dhar P, Wu JD (2018) NKG2D and its ligands in cancer. Curr Opin Immunol 51: 55-61.
- Antonangeli F, Soriani A, Cerboni C, Sciumè G, Santoni A (2017) How mucosal epithelia deal with stress: Role of NKG2D/NKG2D ligands during inflammation. Front Immunol 8: 1583.
- Deng X, Gao F, Li N, Li Q, Zhou Y, et al. (2019) Antitumor activity of NKG2D CAR-T cells against human colorectal cancer cells in vitro and in vivo. Am J Cancer Res 9(5): 945-958.
- Fuertes MB, Domaica CI, Zwirner NW (2021) Leveraging NKG2D ligands in immuno-oncology. Front Immunol 12: 713158.
- Li H, Yang C, Cheng H, Huang S, Zheng Y (2021) CAR-T cells for colorectal cancer: Target-selection and strategies for improved activity and safety. J Cancer 12(6): 1804-1814.
- Townsend MH, Shrestha G, Robison RA, O’Neill KL (2018) The expansion of targetable biomarkers for CAR T cell therapy. J Exp Clin Cancer Res 37(1): 163.
- Cha SE, Kujawski MJ, Yazaki P, Brown C, Shively JE (2021) Tumor regression and immunity in combination therapy with anti-CEA chimeric antigen receptor T cells and anti-CEA-IL2 immunocytokine. Oncoimmunology 10(1): 1899469.
- Zhang C, Wang Z, Yang Z, Wang M, Li S, et al. (2017) Phase I escalating-dose trial of CAR-T therapy targeting CEA+ metastatic colorectal cancers. Mol Ther 25(5):1248-1258.
© 2022. Kim L O’Neill. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






