- Submissions

Full Text
Novel Approaches in Cancer Study
The Calamitous Contagion-Malignant Otitis Externa
Anubha Bajaj*
Histopathologist in A.B. Diagnostics, India
*Corresponding author: Anubha Bajaji, Histopathologist in A.B. Diagnostics, New Delhi, India
Submission: December 13, 2021 Published: January 24, 2022

ISSN:2637-773XVolume6 Issue5
Preface
Preface Malignant otitis externa emerges as a severe, invasive, possibly fatal infection of the external auditory canal. Malignant otitis externa commonly appears as a life-threatening condition in immunocompromised individuals or elderly population with diabetes mellitus. The infection rapidly disseminates within circumscribing soft tissue, temporal bone, base of skull or adjoining neck spaces. Although an infective process, malignant otitis externa functions and disseminates as a malignant neoplasm. Antecedent disease discernment is crucial as the condition is associated with significant proportionate mortality. Malignant otitis external was initially discerned by Meltzer in 1959 and thus scripted by Chandler in 1968 on account of extensive mortality associated with the condition [1]. Malignant otitis externa is additionally designated as necrotizing otitis externa.
Disease Characteristics
Disease Characteristics Malignant otitis externa occurs due to diverse contributory factors such as diabetes mellitus, chronic debilitation or immunosuppression and in subjects on chemotherapy or radiation therapy or elderly individuals. Malignant otitis externa is commonly discerned in elderly subjects above > 60 years although no age of disease emergence is exempt. Malignant otitis externa is infrequent in children [2,3]. Diabetes mellitus and immunosuppression due to chemotherapy or infection with Human Immune Deficiency Virus (HIV) contributes to the development of malignant otitis externa wherein the condition is discerned in younger individuals, the external auditory canal may be devoid of granulation tissue and prognostic outcomes are inferior. Additionally, malignant otitis externa may be discerned in young, non-diabetic or immunocompetent individuals [2,3].
Disease prevalence is declining although zones of enhanced environmental humidity display elevated proportionate infection. A male predominance is observed [2,3]. Majority (~90%) of instances emerge due to infection with pathogenic Pseudomonas aeruginosa. Additionally, organisms such as Proteus mirabilis, Klebsiella spp or Staphylococci may engender the condition. Fungal organisms, especially Aspergillus fumigatus may initiate malignant otitis externa. Intravascular infection may be generated by fungal malignant otitis externa although the temporal bone is usually spared. The condition arises due to tissue ischaemia, neutrophilic migratory defect and virulence of Pseudomonas organism [2,3]. Malignant otitis externa predominantly incriminates the external auditory canal, base of skull, stylomastoid foramen or jugular foramen [2,3]. Bacterial ingress may occur through diverse anatomical routes wherein incrimination of the antero-inferior suprahyoid neck space or masticator space are frequently discerned. Additionally, infection and inflammation may occur through osteo-cartilaginous junction or the osseous canal towards the posterior aspect of the mastoid, temporomandibular joint, parotid gland, anterior cervico-facial spaces or medial aspect with the base of the skull [2,3].
Alternatively, the infection can erode cartilaginous and bony segments of the external auditory canal with consequent, direct intracranial dissemination of infection with severe intracranial complications [3,4]. Disseminated infection initiates bony erosion and infiltrates distant soft tissue through fascial planes and venous sinuses. Infection of the base of skull, temporal bone and circumscribing soft tissue generates invasion of intracranial anatomic structures, stylomastoid foramen, jugular foramen and cranial nerves, such as the facial nerve, glossopharyngeal nerve, vagus nerve and accessory nerve. Dissemination through osseocartilaginous junction and sub temporal area initiates invasion of retro-condylar and para-pharyngeal adipose tissue, temporomandibular joint or the masticator muscle [3,4].
Clinical Elucidation
Clinical Elucidation Malignant otitis externa may commence with features of acute otitis externa followed by pain, purulent otorrhoea, oedema, cellulitis, chondritis or osteomyelitis. Middle ear space or base of the skull may eventually be infected [3,4]. Typical lesions of otitis externa are associated with pain disproportionate to the apparent infection. Besides, pyrexia unresponsive to therapy may appear. The disorder commences with otitis externa and terminates in temporal bone osteomyelitis. Emergence of a mental state indicates development of intracranial complications [3,4]. Malignant otitis externa demonstrates cogent clinical symptoms such as disproportionate pain, oedema, appearance of an exudate, granulation tissue within the external auditory canal and configuration of a post-surgical micro-abscess. Additionally, definitive, disease-specific alterations are observed with technetium-99 (99Tc) bone scan. Localized therapy exceeding (>) one week may be inefficacious [3,4].
Additionally, contributory factors such as diabetes mellitus, incrimination of cranial nerves, specific features on radiography, debilitating physical condition and elderly age are conducive to the development of malignant otitis externa [4,5]. Complications arise with infiltration of circumscribing anatomic structures and cranial nerves. With infection of the sphenoid, occiput and clivus, osteomyelitis of the temporal bone and base of skull may ensue. Intracranial involvement emerges as confusion, meningitis, thrombosis or disease- associated mortality [4,5]. Complications associated with malignant otitis externa appear as osteomyelitis of the temporal bone or base of the skull, subdural empyema, cerebral abscess, meningitis, involvement of temporomandibular joint, thrombophlebitis of the dural venous sinus or erosion at the base of the skull with intracranial dissemination of infection [4,5]. Cranial nerve palsies may ensue wherein the facial nerve is commonly incriminated. Cranial nerves such as facial (VII) nerve, glossopharyngeal (IX) nerve, vagus (X) nerve and accessory (XI) nerve are commonly implicated, whereas the hypoglossal (XII) nerve is exceptionally involved [4,5]. Malignant otitis externa may be associated with inflammation of adjacent anatomic structures such as peri-auricular soft tissues, nasopharynx and parapharyngeal space. Additionally, opacification of mastoid air cells and the middle ear may occur due to direct extension of an infection [5,6]. Elderly subjects depict enhanced possible occurrence of complications and disease-associated mortality [5,6].
Histological Elucidation
Histological Elucidation Upon gross examination, cutaneous ulceration appears adjacent to an osseous segment of the external auditory canal with abundant, subjacent necrotic tissue and granulation tissue [5,6]. Microscopically, superimposed stratified squamous epithelium is necrotic, ulcerated and demonstrates pseudo-epitheliomatous hyperplasia. Subcutaneous tissue is infiltrated by acute and chronic inflammatory cells. Foci of necrotizing vasculitis are observed. Necrotic bone and cartilage are admixed with foci of viable bone which is infiltrated with an intense inflammatory infiltrate. Foci of non-viable bone or cartilage can be discerned as disseminated sequestra [5,6]. The external auditory canal demonstrates ulceration and decimated stratified squamous epithelium along with admixture of bacteria and inflammatory cell exudate entangled within dense fibrous tissue. Epithelial contiguity may be maintained with reactive, mildly hyperplastic epithelium or the occurrence of significant pseudo-epitheliomatous hyperplasia [5,6]. An abscess may be configured along with emergent acute and chronic inflammation. Tissue samples of cartilaginous external auditory canal delineate extension of inflammation into the apo-pilo- sebaceous apparatus [5,6]. Granulation tissue depicts significant proliferation of reactive, capillary-sized, vascular articulations. However, chronic granulomatous inflammation with configuration of epithelioid cell granulomas is absent. Identification of infecting microorganisms may be obtained with pertinent tissue staining. Gram’s stain demonstrates the occurrence of gram-negative bacilli. Polymerase Chain Reaction (PCR) is beneficial in instances with scarce infecting organisms and may be adopted to adequately discern the causative agent [6,7] (Figure 1-8).
Figure 1: Malignant otitis externa demonstrating purulent otorrhoea, oedema and chondritis.
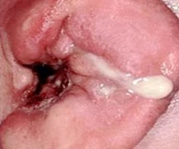
Figure 2: Malignant otitis externa exhibiting purulent discharge, hyperaemia and oedema of the external auditory canal.
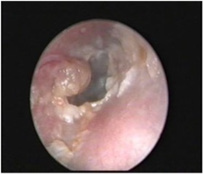
Figure 3: Malignant otitis externa enunciating purulent otorrhoea, hyperaemia and cellulitis.
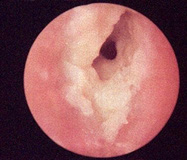
Figure 4: Malignant otitis externa delineating purulent and haemorrhagic ear discharge along with hyperaemia and cellulitis.
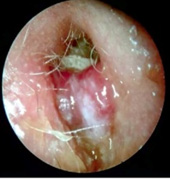
Figure 5: Malignant otitis externa displaying the pertinent site of infection and inflammation as external auditory canal.
Figure 6: Malignant otitis externa depicting aggregates of bacilli intermixed with acute and chronic inflammatory cells.
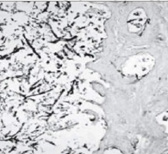
Figure 7: Malignant otitis externa illustrating the infected external auditory canal.
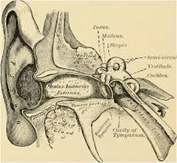
Figure 8: Malignant otitis externa exhibiting accumulation of bacteria with intermingled acute and chronic inflammatory cells.

Differential Diagnosis
Differential Diagnosis Malignant otitis externa requires a segregation from well differentiated squamous cell carcinoma, especially when associated with pseudo-epitheliomatous hyperplasia. The two conditions may concur [6,7]. Besides, malignant otitis externa requires a demarcation from malignant conditions of the external ear. Clinical symptoms can aid in the differentiation. Additionally, malignant otitis externa requires a distinction from conditions such as localized otitis externa, acute diffuse otitis externa, chronic otitis externa, carcinoma of the external auditory canal or osteomyelitis at the skull base due to aspergillosis [6,7].
Investigative Assay
Investigative Assay Upon contrast-enhanced Computerized Tomography (CT), thickened, enhanced soft tissue shadows or erosion of cortical bone appears within the region of the external auditory canal. Configuration of phlegmon or abscess may ensue. Articulation of an abscess is associated with the appearance of a cartilaginous or bony ring with enhancing signal intensities and a necrotic centric zone demonstrating minimal attenuation [8,9]. Technetium-99m bone scan is a sensitive indicator of osteoblastic activity, bone infection and enhanced uptake within the temporal bone and base of the skull, a feature which segregates malignant otitis externa from typical, acute otitis externa. Imaging with gallium- 67 citrate and indium-111 labelled leukocyte scan is beneficial in assessing any response to therapy [8,9]. Total leucocyte count may be normal or mildly elevated. Inflammatory markers are usually elevated and indicate response to antimicrobial therapy. Following adequate disease discernment, Erythrocyte Sedimentation Rate (ESR) and C-Reactive Protein (CRP) require regular evaluation. Erythrocyte Sedimentation Rate (ESR) can be employed to assess response to treatment. Values usually decimate within two weeks of commencing therapy [8,9].
Blood glucose levels requires re-evaluation as baseline glucose intolerance is implicated. Besides, non-diabetics mandate investigation for appearance of diabetes mellitus [8,9]. Appropriate culture of fluid exudate arising from the ear with relevant antibiotic sensitivity is recommended prior to institution of antimicrobial therapy [10,11]. Computerized Tomography (CT) is beneficial in discerning erosion and demineralization of incriminated bone. Generally, obliteration of adipose tissue planes within sub-temporal zones and destruction of bony cortex of mastoid is observed [10,11]. Tissue sampling is optimal in the selection of efficacious antimicrobials, especially in instances of repetitive otitis externa. Tissue samples of external auditory canal are necessitated in order to exclude conditions such as primary malignancy or cholesteatoma [10,11].
Magnetic Resonance Imaging (MRI) is a superior diagnostic tool for discerning anatomical location of malignant otitis externa and associated invasion of soft tissue components. MRI can optimally assess intracranial complications such as thrombosis and intracranial dissemination of infection. Nevertheless, segregation between active inflammation and resolving infection may be challenging. Thus, CT and MRI may not concur with prognostic outcomes of the disease [10,11]. Gallium-67 (Ga67) scan is beneficial in monitoring disease resolution wherein the incriminated area demonstrates enhanced uptake. However, lesion to non-lesion ratio requires assessment for precise evaluation of activity of malignant otitis externa [10,11]. Technetium 99m (Tc99m) scan is advantageous in assessing preliminary disease, although prognostic outcomes may not be appropriately assessed. Besides, nuclear studies may be inadequate in evaluating anatomical extent of disease [10,11]. CT and MRI should be accompanied by Single Photon Emission Computerized Tomography (SPECT) bone imaging for the initial diagnosis of malignant otitis externa. SPECT and gallium-67 scan are optimal methodologies for assaying disease progression [10,11].
Therapeutic Options
Therapeutic Options Cogent antipseudomonal, antibiotic therapy is optimal and recommended. Systemic antimicrobial, antipseudomonal treatment is advantageous in treating instances engendered by Pseudomonas aeruginosa. Pain control and alleviation of accompanying diabetes mellitus is required. Resistant infection can be treated with parenteral, antipseudomonal antibiotics. Appropriate antimicrobial therapy is pertinent to the isolated organism. Duration of therapy relies upon response to therapy and can be terminated following one week of a normal gallium-67 (Ga67) scan [10,11]. Therapeutic efficacy with hyperbaric oxygen remains debatable. Surgical management is recommended in subjects unresponsive to medical therapy. Surgical manoeuvers such as localized debridement of infected tissue, eradication of bony sequestrum and drainage of associated abscess can be employed. However, facial nerve decompression is usually unnecessary in subjects with accompanying facial nerve palsy [10,11].
Malignant otitis externa displays a proportionate reoccurrence of 15% to 20% which may be associated with an elevated Erythrocyte Sedimentation Rate (ESR). Extended disease monitoring of up to one year is recommended. Advent of contemporary treatment options is associated with a decline in disease- associated mortality [10,11]. Prognostic outcome of malignant otitis externa is contingent to duration of diabetes mellitus, levels of Erythrocyte Sedimentation Rate (ESR) and C-Reactive Protein (CRP) along with pertinent imaging features [10,11]. Inferior prognostic outcome is observed in subjects with incrimination of the facial nerve or additional cranial nerves, neurological involvement of non-cranial nerves, extensive granulation tissue or oedema within external auditory canal, bilateral lesions and malignant otitis externa arising due to Aspergillus spp [10,11].
References
- Chandler JR (1968) Malignant external otitis. The Laryngoscope 78 (8): 1257-1294.
- Al Aaraj MS, Kelley C (2021) Malignant Otitis Externa. Stat Pearls International 2021, Treasure Island, Florida, USA.
- van Kroonenburgh AMJL, van der Meer WL, Bothof RJP, van Tilburg M, van Tongeren J, et al. (2018) Advanced imaging techniques in skull base osteomyelitis due to malignant otitis externa. Current Radiology Reports 6(1): 3.
- Hutson KH, Watson GJ (2019) Malignant otitis externa, an increasing burden in the twenty-first century: review of cases in a UK teaching hospital, with a proposed algorithm for diagnosis and management. J Laryngol Otol 133 (5): 356-362.
- Lee SK, Lee SA, Sang WS, Jae HY, Jong DL, et al. (2017) Analysis of prognostic factors in malignant external otitis. Clin Exp Otorhinolaryngol 10(3): 228-235.
- Sylvester MJ, Sanghvi S, Patel V, Eloy JA, Ying YM (2017) Malignant otitis externa hospitalizations: Analysis of patient characteristics. Laryngoscope 127(10): 2328-2336.
- Kaya İ, Sezgin B, Eraslan S, Öztürk K, Göde S, et al. (2018) Malignant otitis externa: A retrospective analysis and treatment outcomes. Turk Arch Otorhinolaryngol 56(2):106-110.
- Manso MC, Rodeia SC, Rodrigues S, Cavilhas P, Domingos R (2016) Malignant otitis externa and stroke. Eur J Case Rep Intern Med 3(4): 000387.
- Mion M, Bovo R, Marchese RM, Martini A (2015) Outcome predictors of treatment effectiveness for fungal malignant external otitis: a systematic review. Acta Otorhinolaryngol Ital 35(5): 307-313.
- Phillips JS, Jones SE (2013) Hyperbaric oxygen as an adjuvant treatment for malignant otitis externa. Cochrane Database Syst Rev (5): CD004617.
- Illing E, Zolotar M, Ross E, Olaleye O, Molony N (2011) Malignant otitis externa with skull base osteomyelitis. J Surg Case Rep (5):6.
© 2022. Anubha Bajaj. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






