- Submissions

Full Text
Novel Approaches in Cancer Study
On The Mechanism of Human Carcinogenesis
Doug Dix*
Department of Health Science and Nursing, USA
*Corresponding author: Doug Dix, Professor of Biology and Medical Technology, Department of Health Science and Nursing, West Hartford, Connecticut-06117, USA
Submission: January 03, 2022 Published: January 12, 2022

ISSN:2637-773XVolume6 Issue5
Opinion
Some bad ideas never die, like drinking and driving, American football, and refusing to take COVID precautions. Thinking that “incidence rates for cancers overall increase steadily as age increases” [1] is such an idea. It originated in the 1950’s [2], but has been thoroughly debunked since then [3,4]. Unfortunately, even the National Cancer Institute continues to promulgate this myth. Look at the age-distribution of new cancer diagnoses in the 2021 NCI report [1]. It is a bar graph, with each bar representing the number of new cancer cases per 100,000 people at each age. The cancer incidence rate does increase with age steadily, meaning regularly or at a relatively unchanging pace, but only from age 20 or so to age 60 or so. After that, the increase in incidence becomes gradually less steady until age 84, after which, incidence declines with advancing age.
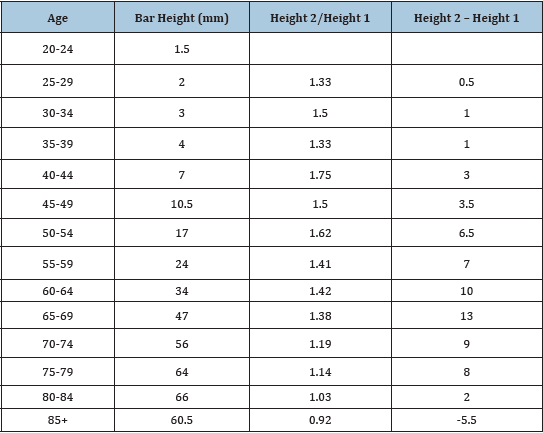
I measured the height of the bars in the above figure and the difference in height between adjacent bars (Table 1). This difference increases steadily to peak at age 65 after which it declines with further advance in age, becoming negative after age 84. The correlation coefficient between this difference in height and age is negative, r =-.18. This should put away, once and for all, the idea that cancer incidence rates overall increase steadily with age. Whatever is responsible for the steady increase in incidence before age 60 cannot be responsible for the steady decline in incidence after that age. Carcinogenesis is not a simple, single mechanism.
Table 1: Age and cancer risk from figure in Reference [1].
Until we put the bad idea away, some cancers seem to exhibit unusual age distributions. Neuroblastoma and retinoblastoma, for instance, reach peak incidence in infancy. Acute lymphoblastic leukemia reaches peak incidence near three years of age, while osteogenic sarcoma does so in adolescence, and testicular cancer and Hodgkin’s lymphoma do so in early adult years. We might imagine the different patterns reflect different mechanisms of carcinogenesis. But once we put the bad idea away, we can see that all cancers studied exhibit a similar age-incidence pattern: A period of low risk followed, after some critical age, by a period of increasing risk at a continuously decreasing rate, followed by a plateau or decline in risk [3,4]. The critical age varies with the tissue of tumor origin. For most cancers, and for all the common cancers, it is after age 40. But the similar pattern for all cancers suggests a similar mechanism of carcinogenesis.
What is that mechanism? It is easier to say what it is not. It is not principally a chance event or any environmentally dependent event. Both of these are inconsistent with the plateau or decline in risk at old ages. By elimination, then, the carcinogenic mechanism must be principally dependent on genes. But twin studies have eliminated segregating gene as the culprits [5]. So, species-specific genes must be responsible. Aging genes are species specific. I suggest these are the principal cause of cancer. They must in some way cause mutations in protooncogenes, tumor suppressor genes, or other critical genes that release mutant cells from mitotic control. To envision a plausible mechanism, I suggest focusing on some key epidemiological findings:
1. In all populations, for all cancers, except those of breast and thyroid, males are at higher risk than females.
2. Males are taller than females, and height generally correlates with cancer risk [6]. Perhaps body height is a surrogate for the size of the stem cell pool in any given tissue. The bigger this pool, the bigger the risk. The larynx is the prime example. It is clearly larger in males as is the cancer risk. Breast and thyroid are the exceptions that may prove the rule. Both are bigger in females than males as is the cancer risk for these tissues.
3. The correlation coefficients between age-standardized incidence rates for pairs of different cancers across populations are only weak or moderate, suggesting that part of the carcinogenic mechanism is determined by chance [6,7]. Perhaps cells are recruited by chance for a potentially carcinogenic event.
4. For the common cancers, the increase in risk between ages 40 and 50 seems pivotal. For the common cancers, the jump in incidence rate between ages 40 and 50 for each cancer correlates strongly with the age-standardized rate for that cancer, and that correlation is stronger than with the jump in incidence rate between any other adjacent ages [6,7]. In addition, this jumps in incidence between ages 40 and 50 correlates strongly with the ASR between genders, races, and ethnicities. Whatever happens to increase risk for the common cancers between ages 40 and 50 seems to determine the total risk of that cancer. I suggest it is the number of mature differentiated cells that enter dedifferentiation.
I propose a theory: During the early low-risk phase of tissue life, stem cells are adequate for tissue maintenance. After some critical age that is specific for each tissue, the original stem cells are exhausted and new stem cells must be created by dedifferentiation of mature cells. This is the potentially carcinogenic event as it enables differentiated cells with damaged DNA to become proliferative. As age advances, senescence gradually takes over and risk declines as a consequence. Anything that accelerates depletion of the original stem cells or increases the need for tissue dedifferentiation, or the risk of mutation increases the risk of cancer. Measures that minimize these events should protect against cancer.
References
- National Cancer Institute (2021) Age and cancer risk.
- Armitage P, Doll R (1957) A two-stage theory of carcinogenesis in relation to the age distribution of human cancer. Brit J Cancer 11(2): 161-169.
- Dix D (1989) The role of aging in cancer incidence. J Gerontol Biol Sci 44(6): 10-18.
- Leipold S, Madeyanda A, Dix D (2001) On the role of aging in carcinogenesis. Anticancer Res 21(6A): 4189-4193.
- Dix D (2003) On the role of genes relative to the environment in carcinogenesis. Mech Ageing Dev 124(3): 323-332.
- Marshall B, Dix D (2017) Human carcinogenesis: Toward a unified theory. Open Access Library J 4(7): e3707.
- Dix D (2019) Human carcinogenesis: The role of age and gender. Anticancer Res 39(8): 4385-4391.
© 2022. Doug Dix. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






