- Submissions

Full Text
Novel Approaches in Cancer Study
Mini Review: Hyperthermia Treatment for Bone Cancers
Justine Katherine Lee and Oana Bretcanu*
School of Engineering, Newcastle University, Newcastle Upon Tyne, UK
*Corresponding author: Oana Bretcanu, School of Engineering, Newcastle University, Newcastle Upon Tyne, NE1 7RU, UK; e-mail: oana.bretcanu@ newcastle.ac.uk
Submission: November 16, 2021 Published: December 08, 2021

ISSN:2637-773XVolume6 Issue4
Abstract
Treatment of bone cancer usually involves surgical resection of the tumours, as well as the use of radiation or drugs, in order to kill tumour cells and/or prevent further growth and reproduction of tumoural cells. Hyperthermia is a complex method of treatment that involves the use of heat to selectively kill tumour cells. Due to their poorly organised blood system, tumours cannot dissipate heat effectively, leading to cell death via different cellular and molecular modifications of the structure and microenvironment of tumour tissue.
Keywords: Cancer; Bone; Hyperthermia
Introduction
Bone is one of the most common sites for metastasis. Approximately 350,000 people die in the United States every year from bone metastases [1]. The most common site of bone metastases is the axial skeleton, with the lumbar region being the most frequently impacted site [2]. Bone cancer can lead to bone pain, hypercalcemia, spinal cord compression and bone fracture, decreasing the quality of life of cancer patients [3]. The most common treatments for bone cancers are surgical resection, radiotherapy and chemotherapy. Hyperthermia is another type of cancer treatment that is generally used for advanced cancers, in combination with radiotherapy or chemotherapy. Clinical trials have shown that hyperthermia reduces the size of tumours, enhancing the effect of radiation or anticancer drugs [4]. Hyperthermia affects both molecular and cellular levels. Alterations to the cytoskeleton and the cell membrane, as well as impairment of protein, Ribonucleic Acid (RNA) and Deoxyribonucleic Acid (DNA) synthesis, inhibition of DNA repair enzymes, altered gene expression and protein denaturation, unfolding and aggregation [5-7] are all changes caused by hyperthermia.
Discussion
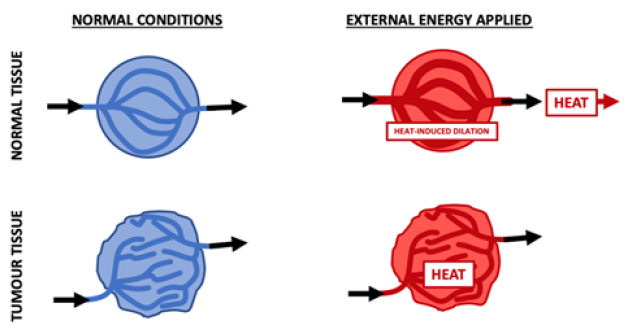
Hyperthermia is defined as ‘raising of cancer tissue to 41 C or higher by external means, with the aim of eradicating active malignant cells to improve cancer control’ [8]. Hyperthermia may selectively target tumour tissue due to its microcirculation differences compared to normal tissue. As tumours have a poorly organised and chaotic blood system, they are highly ineffective at dissipating heat [8]. Following the application of external energy (heat), blood vessels within normal tissue dilate, leading to an increase in blood flow and a subsequent reduction in temperature. However, tumour tissue has a chaotic and poorly organised vasculature, and the vessels cannot dissipate the heat (Figure 1), increasing the temperature locally. This leads to activation of molecular and cellular alterations in the tumour, such as inhibition of DNA repair enzymes and denaturation, unfolding and aggregation of proteins, causing cell death via necrosis or apoptosis [5,7]. Additionally, as the microenvironment of cancerous tissue is characterised by a reduction of blood flow and blood vessel density, it favours hypoxia, acidosis and energy deprivation [7]. Therefore, when compared to normal tissue, greater damage is generated in tumours as a result of hyperthermia [9,10].
Figure 1: Heat dissipation in normal and tumour tissues (adapted from [10]).
Discussion
There are two main types of hyperthermia, thermotherapy
and thermal ablation. Thermotherapy occurs when heat is used
to increase the body temperature to 41-45 C, with the aim of
selectively causing cell death of the tumour, while leaving the
surrounding healthy tissue unaffected. Thermotherapy is also
able to enhance the therapeutic effect of other cancer treatments,
such as radiotherapy and chemotherapy [11]. Conversely, thermal
ablation is the use of temperatures above 45 C in order to cause
destruction of cells. This technique can damage both tumour and
healthy tissue [11].
While the exact mechanism by which hyperthermia induces
cell death is not completely understood, it is thought to be due
to a combination of disruptions that occur within the cell [6].
Nevertheless, hyperthermia ultimately leads to cell death via either
necrosis or apoptosis [7]. Necrosis (premature cell death) is a
passive pathological cell damage that is followed by an inflammatory
response, whereas apoptosis is genetically controlled, programmed
cell death [7]. Apoptosis can occur via the extrinsic or intrinsic
pathways, which differ in the cascade of proteins they activate
although both pathways ultimately lead to cell death [5]. However,
most of the stimuli that cause apoptosis can also induce necrosis
when the cell is subjected to prolonged exposure to heat [7]. It has
been shown that some types of cells show varying susceptibilities
to apoptosis following heat exposure, although above certain
temperatures necrosis is more likely to occur [7].
There are three main categories of hyperthermia: local,
regional and whole-body [6,12]. Local hyperthermia is used for
solid localised tumours that are at or near the surface, and heating
is achieved with the use of external or internal energy sources [6].
Regional hyperthermia is generally utilized when tumours are
located in deep-seated tissues or when larger areas of the body
require heating. This method usually relies on increased perfusion
of organs or limbs through heating of the blood or irrigation of
body cavities [6]. Whole-body treatment is used in order to treat
metastatic cancer and is achieved with the use of a flexible infrared
chamber, a heated blanket, or simply by heating the patient’s
room [6]. More complex methods have been developed that
use approaches such as radiofrequency, microwave, ultrasound
techniques and magnetic fields, which can effectively target deepseated
tissues within the body [6,13,14].
The method by which the body temperature is raised is still
being improved due to challenges that this cancer treatment poses
[15], such as special equipment [4] and its limited ability to directly
target the tumour site [13] or to keep a uniform temperature within
the target area [16]. The effectiveness of the treatment heavily relies
on the duration of the treatment and the temperature obtained
[10,17], as it has been demonstrated that even half a degree rise
in the temperature within the body can have a substantial effect
on cell survival [18]. Therefore, accurate detection is required for
hyperthermia to be successful, as well as the ability to maintain
the temperature throughout the treatment [6]. For example, at
temperatures above 42 C a reduction in blood flow within the
tumour is observed, resulting in impairment of oxygen and nutrient
supply, ultimately leading to acidosis. However, at temperatures
below 42 C improvement of tumour blood flow is detected. This
causes an increase in oxygen content and subsequently improves
the effectiveness of other cancer treatments, such as radiotherapy
and chemotherapy [7].
As well as being able to induce cell death as a singular treatment,
hyperthermia is commonly used as an adjuvant cancer therapy,
due to its ability to enhance the effects of treatments such as
radiotherapy and chemotherapy [10,19]. Results from clinical trials
have shown that there is improved response and survival rates
[7], as well as faster regression rates [20], in patients treated with
both hyperthermia and radiotherapy compared to radiotherapy
alone. Similar results have been observed in patients treated with chemotherapeutics in combination with hyperthermic treatment
versus chemotherapeutics alone [21,22].
The increased effectiveness of radiotherapy, when heat is
applied in conjunction, is most likely a result of an increase in
oxygen levels within the tumour. This leads to the facilitation
of the formation of radiation-induced oxygen radicals, which
produce double-strand breaks in DNA, which may cause cancer
cell destruction [23]. Furthermore, the improved efficacy of
chemotherapeutics when used in combination with hyperthermia
may be due to ‘altered drug pharmacokinetics, such as increased
solubility, altered binding of plasma proteins and activation of
enzymatic processes’ [8]. Additionally, it has been demonstrated
that hyperthermia negatively impacts the DNA repair mechanisms
[8,23], which may play a role in the increased effectiveness of
radiotherapy and chemotherapy.
Conclusion
Hyperthermia is an attractive bone cancer treatment due to its lack of severe side effects. It is mainly used as an adjuvant therapy, in combination with radiotherapy or chemotherapy. However, the use of hyperthermia is currently limited by the ability of existing equipment to effectively target deep-seated tumour sites or maintain a homogeneous temperature within the target area. The development of hyperthermia as a treatment for cancer provides an avenue that allows tumour cells to be destroyed, via the production of heat, with minimal side effects. Due to their poorly organised blood system, tumours are more sensitive to heat than normal tissue. Optimised heat treatment can trigger cancer cell death through numerous cellular and molecular alterations. Development of new materials and minimally invasive devices for localised deepseated bone cancers could unlock the potential of hyperthermia treatment and open it up to wider use.
Conflict of Interest
There is no conflict of interest regarding the publication of this article.
References
- Huang JF, Shen J, Li X, Rengan R, Silvestris N, et al. (2020) Incidence of patients with bone metastases at diagnosis of solid tumors in adults: A large population-based study. Annals of Translational Medicine 8(7): 482.
- Chin H, Kim J (2015) Bone metastasis: Concise overview. Federal Practitioner 32(2): 24-30.
- Apoorva J, Alysia K, Pramod T (2021) Bone metastasis.
- https://www.cancer.gov/about-cancer/treatment/types/hyperthermia
- Ahmed K, Tabuchi Y, Kondo T (2015) Hyperthermia: An effective strategy to induce apoptosis in cancer cells. Apoptosis 20(11): 1411-1419.
- Mallory M, Emile G, Guy CJ, Lester G, Charles BS (2016) Therapeutic hyperthermia: The old, the new, and the upcoming. Critical Reviews in Oncology/Hematology 97: 56-64.
- Hildebrandt B, Peter W, Olaf A, Annette D, Geetha S, et al. (2002) The cellular and molecular basis of hyperthermia. Oncology Hematology 43(1): 33-56.
- Vernon C (1992) Hyperthermia in cancer growth regulation. Biotherapy 4(4): 307-315.
- Song CW (1984) Effect of local hyperthermia on blood flow and microenvironment: A review. Cancer Research 44(10 Suppl): 4721s-4730s.
- Chicheł A, Janusz S, Magda K, Marek K (2007) Hyperthermia - description of a method and a review of clinical applications. Reports of Practical Oncology and Radiotherapy 12(5): 267-275.
- Suriyanto, EYK Ng, Kumar SD (2017) Physical mechanism and modeling of heat generation and transfer in magnetic fluid hyperthermia through Néelian and Brownian relaxation: a review. Bio Medical Engineering Online 16: 1-22.
- Falk M, Issels RD (2001) Hyperthermia in oncology. International Journal of Hyperthermia 17(1): 1-18.
- Deatsch AE, Evans BA (2014) Heating efficiency in magnetic nanoparticle hyperthermia. Journal of Magnetism and Magnetic Materials 354: 163-172.
- Cochis A, Miola M, Bretcanu O, Rimondini L, Vernè E, et al. (2017) Magnetic bioactive glass ceramics for bone healing and hyperthermic treatment of solid tumors, in advanced magnetic and optical materials. In: Ashutosh T, Parameswar K, et al. (Eds.), Scrivener Publishing, Wiley, Beverly, MA, USA, pp. 81-112,
- Wust P, Hildebrandt B, Sreenivasa G, Rau B, Gellermann J, et al. (2002) Hyperthermia in combined treatment of cancer. Lancet Oncol 3(8): 487-497.
- Chatterjee DK, Diagaradjane P, Krishnan S (2011) Nanoparticle-mediated hyperthermia in cancer therapy. Therapeutic Delivery 2(8): 1001-1014.
- Raaphorst GP (1990) Fundamental aspects of hyperthermic biology, in an introduction to the practical aspects of clinical hyperthermia. Taylor and Francis, London, UK, pp. 10-54.
- Chang D, Lim M, Jeroen ACM, Ruirui Q, et al. (2018) Biologically targeted magnetic hyperthermia: Potential and limitations. Frontiers in Pharmacology 9: 1-20.
- Halperin EC, Perez CA, Brady LW (2008) Perez and Brady’s principles and practice of radiation oncology. Wolters Kluwer Health/Lippincott Williams & Wilkins, Philadelphia, USA.
- Kim JH, Hahn EW, Ahmed SA (1982) Combination hyperthermia and radiation therapy for malignant melanoma. Cancer 50(3): 478-482.
- Issels RD, Lars HL, Jaap V, Peter W, Peter R, et al. (2010) Neo-adjuvant chemotherapy alone or with regional hyperthermia for localised high-risk soft-tissue sarcoma: A randomised phase 3 multicentre study. The Lancet Oncology 11(6): 561-570.
- Huilgol NG, Gupta S, Dixit R (2010) Chemoradiation with hyperthermia in the treatment of head and neck cancer. International Journal of Hyperthermia 26(1): 21-25.
- Barry SE (2008) Challenges in the development of magnetic particles for therapeutic applications. International Journal of Hyperthermia 24(6): 451-466.
© 2021. Oana Bretcanu. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






