- Submissions

Full Text
Novel Approaches in Cancer Study
Dendrimer-Based Nanomedicine (Paramagnetic Nanoparticle, Nanocombretastatin, Nanocurcumin) for Glioblastoma Multiforme Imaging and Therapy
Stephen L Brown, James M Snyder and Meser M Ali*
Department of Neurosurgery, USA
*Corresponding author: Meser M Ali, Department of Neurosurgery, Cellular and Molecular Imaging Lab, Detroit, USA
Submission: October 12, 2021 Published: October 20, 2021

ISSN:2637-773XVolume6 Issue3
Abstract
In brain tumors, delivering nanoparticles across the blood-brain tumor barrier presents a major challenge. Dual mode magnetic resonance imaging and fluorescent imaging probes have been developed where relaxation based Gd-DOTA or ParaCEST agents and a Near-Infrared (NIR) fluorescent dye, DL680 were conjugated on the surface of dendrimer. The in vivo and ex vivo imaging of the dual-modality contrast agent showed excellent potential utility for identifying the location of glioma tumors. Systemic delivery of the subsequent nano-sized agent demonstrated glioma-specific accumulation, probably due to the enhanced permeability and retention effect. The biodistribution studies revealed the G5 agents have accumulated in the glioma tumor and the liver while a G3 agent only accumulated in the brain tumor but not in the liver or kidney. Hydrophobic drug molecules like Combrestatin A4 (CA4) or curcumin have also been conjugated with dendrimers that provided high aqueous solubility with improved therapeutic effect.
Keywords: Dendrimer; Nanomedicine; Glioma; Cancer imaging and therapy
Introduction
Glioblastoma Multiforme (GBM, World Health Organization/ WHO grade IV) is the most common malignant primary brain tumor in adults [1]. GBM is the most common primary tumor of the CNS, and accounts for 12-15% of all intracranial tumors and 50-60% of gliomas [2]. GBM is characterized by molecular heterogeneity and the poorest prognosis [2]. Despite the advances made in therapeutic options for GBM, its prognosis remains poor with an overall survival rate (<2 years) that has been stagnant for three decades at approximately 20%. It is one of the unfortunate cancers where no predominant genetic alteration has been identified that could be targeted, resulting in limited therapeutic options. The multimodal treatment of GBM includes maximal surgical resection followed by Radiotherapy (RT) Plus Temozolomide (TMZ) chemotherapy, which may increase median survival to 12-15 months, although the disease typically progresses within 6-9 months, and 2-year survival is less than 25% [3,4].
Invasive biopsy is routinely utilized to assess histological type, classification, grade and potential aggressiveness of brain cancer and also for determination of the type of drug regimen employed for treatment [5,6]. Imaging techniques include Computed Tomography (CT), Positron Emission Tomography (PET), ultrasound and, most importantly, Magnetic Resonance Imaging (MRI) [7-11]. For some brain tumors the delineation of the actual tumor volume is difficult because peritumoral edema does not readily allow precise discrimination of tumor margins [12]. The use of contrast agent helps overcome this deficiency and allows estimates of tumor margins [13-15]. However, such tumor enhancement using contrast agents is possible only in patients with a compromised Blood-Brain Barrier (BBB) [12]. One approach that utilizes the unique structural features of many solid tumors
(hypervasculature, defective vascular architecture, and impaired
lymphatic drainage) [16,17] may lead to relatively selective
extravasation and retention of long circulating nanocarriers. This
phenomenon (“passive targeting”) is essentially the working
principle of most clinically viable targeting strategies based on
nanocarriers. This is the Enhanced Permeation and Retention
(EPR) effect and has been described for nanoparticulate systems
including liposomes, dendrimers, micelles, and polymers [18-20].
Brain tumors demonstrate high levels of angiogenic activity
resulting in formation of torturous and abnormally dilated
vessels with leaky inter-endothelial gaps and fenestration [21-
23]. This hyper-permeable vasculature allows nanoparticles
to extravasate and be retained in tumor interstitium following
systemic administration [24]. Yet, effective transvascular delivery
of nanoparticles across the Blood-Brain Tumor Barrier (BBTB) of
malignant gliomas remains a challenge. We and others recently
developed dendrimer-based paramagnetic nanoparticles that were
found to preferentially accumulate in an orthotopic preclinical
glioma model with a compromised BBTB [23,25,26]. These
dendrimer-based polymers have the advantage of small particle size
[25,26] (∼7-12nm) and therefore the potential to improve tumor
penetration. Drug molecules can also be conjugated on the surface
of dendrimer structure and have the potential for lower systemic
toxicity. Hydrophobic anti-cancer drugs like combretastatin a4
(CA4) and Curcumin (Cur) have been conjugated on the surface of a
Generation 3 (G3) dendrimer that provide high water solubility and
bioavailability with improved therapeutic effects [27,28]. Here we
review the feasibility of dendrimer- based nanomedicine for glioma
imaging and therapy.
Dendrimer-Based Dual Mode Imaging Agent
Poly (Amidoamine) (PAMAM) dendrimers have been widely
used for biomedical applications. This class of polymers has several
favorable properties including a well-defined chemical structure,
globular shape, low polydispersity index, biocompatibility, and
controlled terminal functional groups. Modification of the PAMAM
dendrimer surface functional groups with targeting compounds,
fluorescent groups, and drugs have produced promising imaging and
therapeutic agents [29]. The pharmacokinetics of the dendrimers
have been alerted by conjugating non-scaffold polymers such as
Polyethylene Glycol (PEG). Oxygen rich PEGs interact with water
through hydrogen bonding that minimizes nonspecific interaction
with proteins and other biological molecules in the circulation.
Moreover, the introduction of PEGs on the surface of dendrimers
can reduce the access of the enzymes at the close proximity of the
dendrimer conjugates that provides the stability of the loaded
drugs/genes from in vivo biodegradation [30].
Intravenous administration of a generation five (G5) PAMAM
dendrimer labelled with tritium showed renal-based excretion
[31]. Malik et al. [32] reported that I125-labeled PAMAM dendrimer
demonstrated 60% of dendrimer accumulation in the liver and
only 1% of the injected dose remained in the blood circulation one
hour after administration [32]. In contrast, introduction of PEGs on
the amines surface of PAMAM dendrimer exhibited longer blood
half-life [33]. However, dendrimer-based drug delivery cannot be
expected to be equally effective across tumor types, sizes, locations,
stages and grades. Dendrimers were decorated with relaxationbased
MRI contrast agents [24,25,34,35]. Paramagnetic Chemical
Exchange Saturation Transfer (PARACEST) agents23 and Biosensor
Imaging of Redundant Deviation in Shifts (BIRDS) agents [36-38].
In a recent study a series of PAMAM dendrimer-based Gn-Gd-
DTPA (G1 to G8) were synthesized, and the pharmacokinetics of
the synthesized agents were studied in the BBTB of glioma tumorbearing
rats [24,34,35]. The properties of dendrimer-based Gd-
DTPA agents in vivo depend on the size, core and exterior surface
charge [39,40] and the porosity and pore size of tumor vessels
vary with the type and status of the tumor. It was demonstrated
that gadolinium chelated dendrimer nanoparticles with core sizes
of <12nm permeated the BBTB, whereas larger nanoparticles
were hindered [41]; thus, the upper limit of pore size in the BBTB
of malignant brain tumors is approximately 12nm [24,42,43].
Spherical dendrimer-based paramagnetic nanoparticles ranging
between 4 to 10nm in diameter maintain peak blood concentrations
for several hours [24,41,44]. Gadolinium-based contrast agents
have been widely used as contrast media for MRI. But free Gd3+
ions are toxic to biological systems and a suitable ligand or
chelate must bind the lanthanide to form a bio-unavailable and
nontoxic complex. In 2006, concern associated with the use of
Gd3+ agents were reported due to an apparent link to a disabling
condition known as nephrogenic systemic fibrosis (NSF) [45-47].
Two clinically approved agents - omniscan and magnevist - have
been associated with NSF [47]. These are all linear Gd3+ chelates
based on the structure of Diethylenetriamine Penta-Acetic Acid
(DTPA) which is a less thermodynamically stable linear acyclic
ligand than macrocyclic chelators. Beyond NSF, safety concerns
regarding the retention of Gd3+ in the Central Nervous System
(CNS) have intensified after cases of prolonged signal enhancement
in the brain were reported, particularly in patients experiencing
repeated contrast media administration [48,49]. Therefore, much
attention has been paid to the development of thermodynamically
stable macrocyclic Gd-DOTA based chelates. Recently, a dual
mode - MRI and fluorescence agent has been reported where
thermodynamically stable macrocyclic Gd-DOTA chelates, and a
fluorophore (dylight680) have been conjugated with a Generation 5
(G5) dendrimer (Figure 1) [25]. The pharmacokinetics of GdDOTAG5-
DL680 was studied in an experimental rat model of glioma with
MRI monitoring [25]. The intravenous delivery of (GdDOTA)54-G5-
DL680 led to the visualization of the agent in the rat glioma tumor.
In another report, a Near Infrared Red (NIR) dye, DyLight680
(DL680) was also conjugated with a dendritic PARACEST
(Paramagnetic Chemical Exchange Saturation Transfer) agent
to detect glioma in vivo in a compromised BBTB.23 Intravenous
delivery of the designed nanomedicine in Figure 1 [(GdDOTA)54-
G5-DL680], resulted in the agent homing into its glioma tumor site
selectively. In vivo MRI detected the agent in a glioma tumor, but not
in contralateral tissue.
Figure 1: Schematic view of dual-mode dendritic conjugate [(GdDOTA)54-G5- DL680]. MRI contrast agent, Gd- DOTA is conjugated with a G5 PAMAM dendrimer. A fluorescent dye, DyLight (DL680), is also conjugated with the same dendrimer.
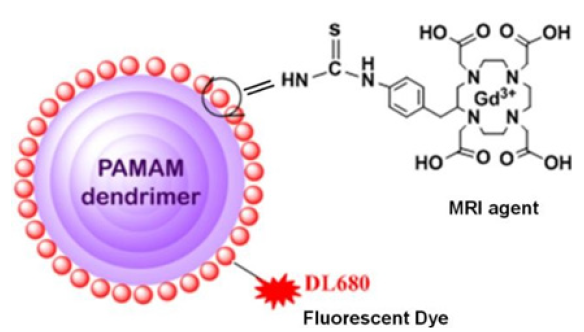
The specificity of the agent was validated by whole-body
NIR-optical imaging and ex vivo fluorescence imaging. The in
vivo MRI showed the macroscopic location of the tumor while
fluorescence imaging showed the biodistribution of the agent.
A dual mode MRI-optical approach is ideally suited for in vivo
biomedical imaging because MRI provides non-invasive in vivo
high-resolution anatomical images, while fluorescence imaging
has high sensitivity and can provide microscopic information in
ex-vivo pathological tissues. BIRDS is a type of molecular imaging
platform for magnetic resonance that utilizes the unique properties
of low molecular weight paramagnetic monomers by detecting
hyperfine-shifted nonexchangeable protons and transforming the
chemical shift information to reflect its microenvironment (e.g.,
via temperature, pH, etc.). Current BIRDS studies have only used
low molecular weight paramagnetic monomers (e.g., Ln-DOTP5-,
Ln-DOTMA-, Ln-DOTA-4AmP5-) [36-38]. Despite their capability to
accurately report molecular readouts (e.g., temperature, pH, etc.),
BIRDS studies with the low molecular weight monomers suffer
from short blood half-life and wide in-vivo distribution. To further
improve the translational potential of BIRDS, we have conjugated
thermodynamically stable macrocyclic p-SCN-Bn-DOTA to the
amines on the surface of PAMAM dendrimers from generation 1
(G1) to generation 4 (G4) by using an isothiocyanatobenzyl group
that achieves excellent synthesis yield (i.e., greater than 60%) and
purities [50]. Dendrimer-based BIRDs agents demonstrated both
acidic pH and temperature-sensing properties [50]. Therefore,
we hypothesize that dendrimeric BIRDS agents have potential
for a range of biomedical applications in quantitative theranostic
imaging.
Nanodrug carriers attached to surface ligands or antibodies
exploit the receptor-mediated uptake pathways that are recognized
by the tumor cells [51-55]. Transferrin receptor-targeting
dendrimers have demonstrated efficiency in delivering therapeutic
genes and drugs to cancer cells and their use has already led to
significant improvements in cancer therapies [56,57].
Dendrimer-Based Drug Conjugate
The vasculature of GBM is fundamentally different from that
of normal vasculature and offers a unique target for anti-cancer
therapy. Therefore, direct targeting of tumor vasculature with
vascular disrupting agents (VDAs) is distinctly different from antiangiogenic
strategies and offers a complementary approach to
standard therapies. Combretastatin A4 (CA4) is a potent vascular
disrupting drug but insoluble in water. CA4 was conjugated with a
G3-succinamic acid PAMAM dendrimer. Conjugation of CA4 with G3
dendrimer improved water solubility as well as bioavailability [28].
However, intravenous (i.v.) delivery of G3-CA4 in an orthotopic
glioma model induced necrosis at the core of the tumor leaving a
rim of viable tissue. By applying the designed dendrimer-based
drug conjugate strategy and tumor-specific prodrug activation
mechanism, we observed the true success of inducing necrosis at
the core of the tumor in an orthotopic U-251 glioma preclinical
animal model [28]. Curcumin (Cur), a yellow pigment in the spice
turmeric (curcuma longa), has been reported for its potential
chemo preventive and chemotherapeutic activity by influencing
various processes, such as cell cycle arrest, differentiation, and
apoptosis in a series of cancers [58-65]. In addition, Cur inhibited
proliferation, migration and invasion of GBM cells in in-vitro studies
[66]. Nevertheless, a major criticism of Cur has been the apparent
poor systemic bioavailability in in-vivo animal models. The latter
indicates poor relevance for clinical translation even when patients
are given up to 8-10grams of the free drug orally each day [63]. In
addition, systemic delivery of Cur leads to non-specific distribution
throughout the body [67]. It is reasonable to explore novel
formulations of Cur that overcome the limitations mentioned above.
A Generation 3 (G3) PAMAM dendrimer-based Cur conjugate was synthesized [27]. The synthesized G3-Cur conjugate demonstrated
full solubility in aqueous media.
The in vitro study revealed that G3-Cur nanoparticles were
internalized into glioma U-251 cells. Systemic delivery of G3-
Cur conjugate led to preferential accumulation in an orthotopic
preclinical glioma model and minimized systemic toxic effects. A
dendrimer based G5 dual mode probe accumulated to GBM tumor
and liver, respectively [25]. G3-Cur nanoparticles showed more
accumulation in tumor and/or less renal uptake, likely due to small
particle size, optimal surface charge, and hydrophilicity in blood
[27]. Multicolor microscopy images of the tumor tissue showed that
G3-Cur particles were internalized inside tumor cells selectively
and further localized within nuclei. It has been demonstrated that
after oral administration of 400mg of curcumin to rats only traces
of unchanged drug were found in the liver and kidney. At 30 min,
90% of curcumin was found in the stomach and small intestine,
but only 1% was present at 24h [68]. While Cur has shown a wide
range of non-specific in vivo distribution, [63]G3-Cur preferentially
accumulated at the tumor site. Enhanced bioavailability of G3- Cur
conjugate was also observed with improved therapeutic efficacy
against cells from different types of cancer. The specificity of G3-
Cur was further investigated with ex vivo multicolor fluorescence
microscopy, which showed accumulation of the particles in
glioma tumor tissue selective for nuclear distribution. Ex vivo
fluorescence microscopy showed that the G5 agent accumulated in
the glioma tumor and the liver [23,25]. Therefore, conjugation of
Cur to a smaller size G3 carrier improved bioavailability and tumor
targeting [27].
Conclusion
The overall ineffectiveness of small molecule chemotherapy drugs in treating malignant brain tumors can be attributed to the fact that there is only a transient elevation in drug concentrations within the extravascular extracellular compartments of tumor tissue due to the short blood half-life of small molecule chemotherapy [24]. We and others have developed dendrimer-based paramagnetic nanoparticles that cross the BBTB and accumulate in the GBM tumor site [23,69-71]. Overexpressed cell surface receptors or the process of tumor acidosis are targeted with high affinity ligands. The result demonstrates the relative accumulation of the nanomedicine to the tumor site. The delivery vehicle introduced here can be loaded with imaging agents in combination with a particular drug, thus offering the possibility of developing a nanotheranostic approach to the treatment of GBM.
Acknowledgement
The authors acknowledge research support from the National Institutes of Health (NIH) grant RO1CA206190 to MMA.
References
- Johnson DR, O'Neill BP (2012) Glioblastoma survival in the United States before and during the temozolomide era. Journal of Neuro-Oncology 107(2): 359-364.
- Hess KR, Broglio KR, Bondy ML (2004) Adult glioma incidence trends in the United States, 1977- 2000. Cancer 101(10): 2293-2299.
- Wen PY, Kesari S (2008Z) Malignant gliomas in adults. The New England Journal of Medicine 359(5): 492-507.
- Dhermain F, Ducreux D, Bidault F, Bruna A, Parker F, et al. (2005) Use of the functional imaging modalities in radiation therapy treatment planning in patients with glioblastoma. Bulletin Du Cancer 92(4): 333-342.
- Becker CM, Beaudry P, Funakoshi T, Benny O, Zaslavsky A, et al. (2011) Circulating endothelial progenitor cells are up- regulated in a mouse model of endometriosis. The American Journal of Pathology 178(4): 1782-1791.
- Chen AC, Wu MH, Chang CH, Cheng CY, Hsu KY (2011) Single portal endoscopic carpal tunnel release: Modification of Menon's technique and data from 65 cases. International Orthopaedics 35(1): 61-65.
- Wintermark M, Sincic R, Sridhar D, Chien JD (2008) Cerebral perfusion CT: technique and clinical applications. Journal of Neuroradiology 35(5): 253-260.
- Tovi M (1993) MR imaging in cerebral gliomas analysis of tumour tissue components. Acta Radiologica Supplementum 384: 1-24.
- Hanson MW, Glantz MJ, Hoffman JM, Friedman AH, Burger PC, et al. (1991) FDG-PET in the selection of brain lesions for biopsy. Journal of Computer Assisted Tomography 15(5): 796-801.
- Pichler A, Prior JL, Piwnica-Worms D (2004) Imaging reversal of multidrug resistance in living mice with bioluminescence: MDR1 P-glycoprotein transports coelenterazine. Proceedings of the National Academy of Sciences of the United States of America 101(6): 1702-1707.
- Boekelheide K, Lee J, Shipp EB, Richburg JH, Li G (1998) Expression of Fas system-related genes in the testis during development and after toxicant exposure. Toxicology Letters 102-103: 503-508.
- Shamji MF, Fric-Shamji EC, Benoit BG (2009) Brain tumors and epilepsy: pathophysiology of peritumoral changes. Neurosurgical Review 32(3): 275-284.
- Lehmann P, Vallee JN, Saliou G, Monet P, Bruniau A, et al. (2009) Dynamic contrast-enhanced T2*-weighted MR imaging: A peritumoral brain oedema study. Journal of Neuroradiology 36(2): 88-92.
- Pronin IN, McManus KA, Holodny AI, Peck KK, Kornienko VN (2009) Quantification of dispersion of Gd-DTPA from the initial area of enhancement into the peritumoral zone of edema in brain tumors. Journal of Neuro-Oncology 94(3): 399-408.
- Schoenegger K, Oberndorfer S, Wuschitz B, Struhal W, Hainfellner J, et al. (2009) Peritumoral edema on MRI at initial diagnosis: An independent prognostic factor for glioblastoma? European Journal of Neurology 16(7): 874- 878.
- Matsumura Y, Maeda H (1986) A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Research 46(12 Pt 1): 6387-6392.
- Maeda H, Wu J, Sawa T, Matsumura Y, Hori K (2000) Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. Journal of Controlled Release 65(1-2): 271-284.
- Chang WT, Pan CY, Rajanbabu V, Cheng CW, Chen JY (2011) Tilapia (Oreochromis mossambicus) antimicrobial peptide, hepcidin 1-5, shows antitumor activity in cancer cells. Peptides 32(2): 342-352.
- Borner MM (2006) Molecular targets in colon cancer. Therapeutische Umschau Revue Therapeutique 63(4): 243-248.
- Fujisawa H, Koide N, Kono T, Takayama K, Tsukioka K, et al. (2002) Expression of basic fibroblast growth factor and its receptor-1 in cardiac myxoma. The Journal of Cardiovascular Surgery 43(5): 589-594.
- Veiseh O, Sun C, Fang C, Narayan B, Gunn J, et al. (2009) Specific targeting of brain tumors with an optical/magnetic resonance imaging nanoprobe across the blood-brain barrier. Cancer Research 69(15): 6200-6207.
- Jain R, Ellika SK, Scarpace L, Schultz LR, Rock JP, et al. (2008) Quantitative estimation of permeability surface-area product in astroglial brain tumors using perfusion CT and correlation with histopathologic grade. AJNR American Journal of Neuroradiology 29(4): 694-700.
- Akcay C, Tom ME, Campbell SE, Beecher MD (2013) Song type matching is an honest early threat signal in a hierarchical animal communication system. Proceedings Biological sciences / The Royal Society 280(1756): 20122517.
- Sarin H, Kanevsky AS, Wu H, Kyle RB, Fung SH, et al. (2008) Effective transvascular delivery of nanoparticles across the blood-brain tumor barrier into malignant glioma cells. Journal of Translational Medicine 6: 80.
- Karki K, Ewing JR, Ali MM (2016) Targeting glioma with a dual mode optical and paramagnetic nanoprobe across the blood-brain tumor barrier. J Nanomed Nanotechnol 7(4): 395.
- Sarin H (2010) On the future development of optimally sized lipid-insoluble systemic therapies for CNS solid tumors and other neuropathologies. Recent Patents on CNS Drug Discovery 5(3): 239-252.
- Gamage NH, Jing L, Worsham MJ, Ali MM (2016) Targeted theranostic approach for glioma using dendrimer-based curcumin nanoparticle. J Nanomed Nanotechnol 7(4): 393.
- Gonawala S, Aryal M, Ewing JR, deCarvalho AC, Kalkanis S, et al. (2018) MRI monitoring of cerebral blood flow after the delivery of nanocombretastatin across the blood brain tumor barrier. J Nanomed Nanotechnol 9(5): 516.
- Luong D, Kesharwani P, Deshmukh R, Cairul M, Umesh G, et al. (2016) PEGylated PAMAM dendrimers: Enhancing efficacy and mitigating toxicity for effective anticancer drug and gene delivery. Acta Biomaterialia 43: 14-29.
- Kaminskas LM, Boyd BJ, Porter CJ (2011) Dendrimer pharmacokinetics: the effect of size, structure and surface characteristics on ADME properties. Nanomedicine (Lond) 6(6): 1063-1084.
- Kukowska-Latallo JF, Candido KA, Cao Z, Shraddha N, Istvan M, et al. (2005) Nanoparticle targeting of anticancer drug improves therapeutic response in animal model of human epithelial cancer. Cancer Research 65(12): 5317-5324.
- Malik N, Wiwattanapatapee R, Klopsch R, Lorenz K, Frey H, et al. (2000) Dendrimers: Relationship between structure and biocompatibility in vitro, and preliminary studies on the biodistribution of 125I-labelled polyamidoamine dendrimers in vivo. Journal of Controlled Release 65(1-2): 133-148.
- Padilla De Jesus OL, Ihre HR, Gagne L, Frechet JM, Szoka FC (2002) Polyester dendritic systems for drug delivery applications: In vitro and in vivo Bioconjugate Chemistry 13(3): 453-461.
- Sarin H, Kanevsky AS, Fung SH, Butman J, Cox R, et al. (2009) Metabolically stable bradykinin B2 receptor agonists enhance transvascular drug delivery into malignant brain tumors by increasing drug half-life. Journal of Translational Medicine 7: 33.
- Sarin H (2010) Physiologic upper limits of pore size of different blood capillary types and another perspective on the dual pore theory of microvascular permeability. Journal of Angiogenesis Research 2: 14.
- Huang Y, Coman D, Ali MM, Hyder F (2015) Lanthanide ion (III) complexes of 1,4,7,10- tetraazacyclododecane-1,4,7,10-tetraaminophosphonate for dual biosensing of pH with Chemical Exchange Saturation Transfer (CEST) and Biosensor Imaging of Redundant Deviation in Shifts (BIRDS). Contrast Media & Molecular Imaging 10(1): 51-58.
- Coman D, Kiefer GE, Rothman DL, Sherry AD, Hyder F (2011) A lanthanide complex with dual biosensing properties: CEST (Chemical Exchange Saturation Transfer) and BIRDS (biosensor Imaging of redundant Deviation in Shifts) with europium DOTA-tetraglycinate. NMR in Biomedicine 24(10): 1216-1225.
- Coman D, de Graaf RA, Rothman DL, Hyder F (2013) In vivo three-dimensional molecular imaging with Biosensor Imaging of Redundant Deviation in Shifts (BIRDS) at high spatiotemporal resolution. NMR in Biomedicine 26(11): 1589-1595.
- Kobayashi H, Brechbiel MW (2003) Dendrimer-based macromolecular MRI contrast agents: characteristics and application. Molecular Imaging 2(1): 1-10.
- Sato N, Kobayashi H, Hiraga A, Saga T, Konishi J, et al. (2001) Pharmacokinetics and enhancement patterns of macromolecular MR contrast agents with various sizes of polyamidoamine dendrimer cores. Magnetic Resonance in Medicine 46(6): 1169-1173.
- Sarin H (2009) Recent progress towards development of effective systemic chemotherapy for the treatment of malignant brain tumors. Journal of Translational Medicine 7: 77.
- Sonavane G, Tomoda K, Makino K (2008) Biodistribution of colloidal gold nanoparticles after intravenous administration: effect of particle size. Colloids and Surfaces B, Biointerfaces 66(2): 274-280.
- Binion DG, Otterson MF, Rafiee P (2008) Curcumin inhibits VEGF-mediated angiogenesis in human intestinal microvascular endothelial cells through COX-2 and MAPK inhibition. Gut 57(11): 1509-1517.
- Sarin H (2010) Overcoming the challenges in the effective delivery of chemotherapies to CNS solid tumors. Therapeutic Delivery 1(2): 289-305.
- Grobner T (2006) Gadolinium-a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrology, Dialysis, Transplantation 21(4): 1104-1108.
- Marckmann P, Skov L, Rossen K, Dupont A, Damholt M, et al. (2006) Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. Journal of the American Society of Nephrology 17(9): 2359-2362.
- Kurtkoti J, Snow T, Hiremagalur B (2008) Gadolinium and nephrogenic systemic fibrosis: Association or causation. Nephrology (Carlton) 13(3): 235-241.
- Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D (2014) High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: Relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 270(3): 834-841.
- Kanda T, Fukusato T, Matsuda M, Toyoda K, Oba H, et al. (2015) Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: Evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology 276(1): 228-232.
- Chang CY, Cheng TJ, Chang FR, Wang HY, Kan WC, et al. (2011) Macrophage mediated anti-proliferation effects of Anthodia camphorata non-polysaccharide-based extracts on human hepatoma cells. Bioscience, Biotechnology, and Biochemistry 75(4): 624-632.
- Cirstoiu-Hapca A, Bossy-Nobs L, Buchegger F, Gurny R, Delie F (2007) Differential tumor cell targeting of anti-HER2 (Herceptin) and anti-CD20 (Mabthera) coupled nanoparticles. International Journal of Pharmaceutics 331(2): 190-196.
- Rapoport N, Gao Z, Kennedy A (2007) Multifunctional nanoparticles for combining ultrasonic tumor imaging and targeted chemotherapy. Journal of the National Cancer Institute 99(14): 1095-1106.
- Ponce AM, Vujaskovic Z, Yuan F, Needham D, Dewhirst MW (2006) Hyperthermia mediated liposomal drug delivery. International Journal of Hyperthermia 22(3): 205-213.
- Ali MM, Woods M, Suh EH, Kovacs , Tircso G, et al. (2007) Albumin-binding PARACEST agents. Journal of Biological Inorganic Chemistry 12(6): 855-865.
- Ahn BH, Lee JH, Bae YS (2003) Identification of mutations in protein kinase CKIIbeta subunit that affect its binding to ribosomal protein L41 and homodimerization. Journal of Biochemistry and Molecular Biology 36(4): 344-348.
- Han L, Huang R, Li J, Liu S, Huang S, et al. (2011) Plasmid pORF-hTRAIL and doxorubicin co- delivery targeting to tumor using peptide-conjugated polyamidoamine dendrimer. Biomaterials 32(4): 1242-1252.
- Li Y, He H, Jia X, Lu WL, Lou J (2012) A dual targeting nanocarrier based on poly(amidoamine) dendrimers conjugated with transferrin and tamoxifen for treating brain gliomas. Biomaterials 33(15): 3899-3908.
- Cho YA, Lee W, Choi JS (2012) Effects of curcumin on the pharmacokinetics of tamoxifen and its active metabolite, 4-hydroxytamoxifen, in rats: possible role of CYP3A4 and P-glycoprotein inhibition by curcumin. Die Pharmazie 67(2): 124-130.
- Choi BH, Kim CG, Bae YS, Lim Y, Lee YH, et al. (2008) p21 Waf1/Cip1 expression by curcumin in U-87MG human glioma cells: Role of early growth response-1 expression. Cancer Research 68(5): 1369-1377.
- Choi BH, Kim CG, Lim Y, Shin SY, Lee YH (2008) Curcumin down-regulates the multidrug- resistance mdr1b gene by inhibiting the PI3K/Akt/NF kappa B pathway. Cancer Letters 259(1): 111-118.
- Choi H, Chun YS, Kim SW, Kim MS, Park JW (2006) Curcumin inhibits hypoxia-inducible factor- 1 by degrading aryl hydrocarbon receptor nuclear translocator: A mechanism of tumor growth inhibition. Molecular Pharmacology 70(5): 1664-1671.
- Choudhuri T, Pal S, Das T, Sa G (2005) Curcumin selectively induces apoptosis in deregulated cyclin D1-expressed cells at G2 phase of cell cycle in a p53-dependent manner. The Journal of Biological Chemistry 280(20): 20059-20068.
- Yallapu MM, Jaggi M, Chauhan SC (2012) Curcumin nanoformulations: A future nanomedicine for cancer. Drug Discovery Today 17(1-2): 71-80.
- Yang CL, Liu YY, Ma YG, Xue Y, Liu D, et al. (2012) Curcumin blocks small cell lung cancer cells migration, invasion, angiogenesis, cell cycle and neoplasia through Janus kinase-STAT3 signalling pathway. PloS One 7(5): e37960.
- Yang CW, Chang CL, Lee HC, Chi CW, Pan JP, et al. (2012) Curcumin induces the apoptosis of human monocytic leukemia THP-1 cells via the activation of JNK/ERK pathways. BMC Complementary and Alternative Medicine12: 22.
- Senft C, Polacin M, Priester M, Seifert V, Kogel D, et al. (2010) The nontoxic natural compound Curcumin exerts anti-proliferative, anti-migratory, and anti-invasive properties against malignant gliomas. BMC Cancer 10: 491.
- Yallapu MM, Othman SF, Curtis ET, Bauer N, Neeraj C, et al. (2012) Curcumin-loaded magnetic nanoparticles for breast cancer therapeutics and imaging applications. International Journal of Nanomedicine 7: 1761-1779.
- Ravindranath V, Chandrasekhara N (1980) Absorption and tissue distribution of curcumin in rats. Toxicology 16(3): 259-265.
- Ali MM, Liu G, Shah T, Flask CA, Pagel MD (2009) Using two chemical exchange saturation transfer magnetic resonance imaging contrast agents for molecular imaging studies. Accounts of Chemical Research 42(7): 915-924.
- Ali MM, Janic B, Babajani-Feremi A, Varma RS, Iskander ASM, et al. (2010) Changes in vascular permeability and expression of different angiogenic factors following anti-angiogenic treatment in rat glioma. PloS One 5(1): e8727.
- Burgos A, Kaplan R, Rodríguez N, Vetanzo M, Morelatto R, et al. (2008) Malignant melanoma of the oral cavity. Rev Fac Cien Med Univ Nac Cordoba 65(2): 70-73.
© 2021. Meser M Ali. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






