- Submissions

Full Text
Novel Approaches in Cancer Study
Analysis of Thrombosis Risk Stratification Models Based on 10 Years Follow up of 237 Essential Thrombocythemia Patients
Adam Kellner1, Vasana Kellner1, Peter Rajnics1, Eva Karádi1, Arpad Illes2, Miklos Udvardy2, Lajos Homor3, Peter Dombi4, Jozsef Herczeg1, Zoltan Sipiczki5, Viktoria Gyorine Korom1, and Miklos Egyed1
1Department of Hematology, Hungary
2Department of Hematology, Hungary
3Faculty of Humanities and Social Sciences, Hungary
4Department of Hematology, Hungary
*Corresponding author:Miklos Egyed, Department of Haematology, Tallián Gyulautca 20-32, Kaposvár, 7400, Hungary
Submission: November 20, 2020 Published: January 22, 2021

ISSN:2637-773XVolume5 Issue5
Abstract
Objective: In our study we analysed thromboembolic (TE) events of ET patients using data from the Hungarian Myeloproliferative Neoplasm (HUMYPRON) registry. We examined possible thrombosis risk factors and compared Landolfi-, IPSET- and R-IPSET risk stratification models.
Methods: The HUMYPRON registry was estabilished in 2012 and contains data of patients from 14 hungarian haematology centers. We analysed clinical and laboratory data of 237 ET (according to 2008 WHO classification) patients to assess possible risk factors of post-diagnostic TE events and to study the applicability of Landolfi-, IPSET- and R-IPSET thrombosis risk stratifications.
Results: 237 ET patients were followed for 10 years on average. After diagnosis 76 patient (32.1%) had TE complication. Previous thrombotic episode was found to be the only factor that had significant effect on TE events after diagnosis (p<0.001). There were marginally less TE events after diagnosis in case of patients who had extremely high (≥1.000x109/L) platelet count measured at the time of diagnosis (p=0.047). On our sample R-IPSET proved to be the strongest model, where the frequency of TE events was 14.3% in the very low and 46% in the high-risk patient group.
Conclusions: During the follow-up period of 10 years on average, prior TE event was the only factor that had highly significant effect on post-diagnostic thrombosis. Among the risk stratification systems R-IPSET model proved to be the strongest.
Keywords: Essential thrombocythaemia;Thrombosis;Humypron registry;Risk factors; Landolfi; IPSET; R-IPSET
Abbreviations: AMIN:Minor Arterial TE Event; AMAJ: Major Arterial TE Event; VMAJ:Major Venous TE Event; ANA:Anagrelide; HU:Hydroxyurea; ASA:Acetylsalicylic acid
Introduction
Essential Thrombocythaemia (ET) is a clonal stem cell disease that belongs to the Philadelphia Negative Chronic Myeloproliferative Neoplasms (MPN) and is characterized by megakaryocytosis of the bone marrow and thrombocytosis. The incidence of the disease in western European countries is 0.2-2.5/100,000 people, its prevalence is 38-57/100,000 [1,2]. The average age at diagnosis is 60 years, and the incidence is twice as high in women than in men [3]. The diagnosis of ET is currently established according to 2016 WHO criteria [4,5]. There is no specific molecular or genetic marker for diagnosis of the disease. The most common alteration is the presence of Janus kinase 2 (JAK2) V617F activation mutation, which occurs in about 50-60% of the cases [5-7]. Calreticulin (CALR) mutation is present in 15-32% of patients, and myeloproliferative leukemia virus oncogene (MPL) mutation appears in 3-4% [5-7]. 10-20% of all cases is triple negative, where none of these mutations can be detected [6-8].
The median survival for the entire patient population is 20 years, but for those younger than 60 this value is already 30 years [8]. Morbidity and mortality of patients are primarily determined by thrombotic and haemorrhagic events. There are significant differences in the incidence of thrombohaemorrhagic events in the literature, which may be resulted by differences in event definition, patient selection, and applied
therapy. In a study of 100 patients, thrombotic events occurred
at a rate of 6.6%/patient-year and haemorrhagic events at a
frequency of 0.33%/patient-year [9]. In another report, the
incidences of thrombotic and bleeding events were 8.1%/patientyear
and 2.5%/patient-year respectively [10]. Transformation into
myelofibrosis occurs in 0.8-4.9% in 10 years and 4-11% in 15 years
[8]. Transformation into acute myeloid leukaemia is observed in
0.7-3% in 10 years and 2.1-5.3% in 15 years [8].
Because of the very good patient survival and the lack of
evidence for life prolongation of any kind of medication, recent
therapy of ET focuses on prevention of the thrombo hemorrhagic
events. Identification of risk groups and their appropriate
management are highly important tasks. There are several risk
stratifications for thrombosis. The IPSET-thrombosis (International
Prognostic Score for Thrombosis in Essential Thrombocythemia)
risk stratification takes age, previous thrombosis, JAK2 positivity
and cardiovascular risk factors into account [11]. In this score
system, the pre-diagnostic thrombosis and JAK2 positivity are more
heavily weighted. Landolfi categorizes patients into low, medium,
high, and extreme high-risk groups based on age, pre-thrombotic
events, white blood cell and platelet count, and general vascular risk
factors [12]. In his therapeutical recommendation, acetylsalicylate
(ASA) treatment is sufficient for patients at low to moderate risk
for thrombosis, whereas cytoreductive therapy is recommended for
patients at high and extreme risk.
The revised IPSET model for thrombosis (R-IPSET) proposed
by Tefferi distinguishes four groups, very low, low, medium, and
high risk, based on age, prior TE event, and JAK2/MPL mutation
[13]. Cardiovascular (CV) risk factors are not included in his
risk assessment of thrombosis but are taken into account at the
therapeutic recommendation. Accordingly, it is not necessary to
treat patients at very low risk for thrombosis who do not have a
cardiovascular risk factor. While, in presence of CV risk factor he
recommends 1x100 mg ASA per day. Low-risk patients are advised
to take 1x100mg or 2x100mg ASA daily depending on CV risk
factors. He warns of giving ASA for those with an extremely high
platelet count (≥1.000x109/l) due to increased bleeding tendency.
For patients at moderate risk of thrombosis who have no CV risk
factor, 1x100mg ASA is suggested on a daily basis, while in the
presence of CV risk factor hydroxyurea is needed in addition to
ASA. Patients at high risk for thrombosis, depending on whether
they had an arterial or venous TE event, are recommended to
receive 2x100mg ASA or systemic anticoagulation in addition to
hydroxyurea.
Landolfi and Tefferi both attribute significant importance to
previous TE events, and when they occur, the patient is classified
into high-risk category. The Hungarian MPN Working Group
(HUMYPRON GROUP) is an online registry that contains clinical
and laboratory data of patients with myeloproliferative neoplasms
[14,15]. In our study we compared the usability of these three risk
stratifications on the ET patient group of the HUMYPRON database
with a follow up period of 10 years.
Methods
Subjects and data collection
The HUMYPRON registry was founded in 2012 and contains data of 237 ET patients from 14 hungarian haematology centers. The diagnosis of ET was established according to the 2008 World Health Organization criteria. The details of study methods have been published before [14]. In brief, clinicians completed a questionnaire that focuses on risk stratification, treatment and complications. As for thrombotic risk stratification Landolfi-, IPSET- and R-IPSET scoring systems were used. Data of ET patients was analysed retrospectively with a cut-off date of December 2018. Based on the database we examined potential risk factors of thrombosis and compared Landolfi-, IPSET- and R-IPSET risk stratification systems. The aim of the present study was to determine the risk stratification that proved to be the most useful when examining this group of patients.
Definition of thrombotic events
Thrombotic events were defined according to Gisslinger [16]:
1. Major arterial thrombosis: stroke, myocardial infarction,
peripheral arterial thrombosis and splanchnic arterial
thromboembolism.
2. Minor arterial thrombosis: TIA, angina pectoris, unstable
angina, generalized convulsion, erythromelalgia, ocular
symptoms, angina abdominalis (transient abdominal
ischemia).
3. Major venous events: deep venous thrombosis, pulmonary
embolism, splanchnic venous thrombosis, and other major
venous events.
Minor venous events were not considered in this study. When
a patient suffered more thromboembolic episodes of the same
phenotype, we only regarded it once.
Treatment
ET patients of our study received either anagrelide or hydroxiurea+acetylsalicilic acid therapy. Those who switched from one type of medication to the other were excluded from the analysis. Because of platelet aggregation inhibition caused by the blocking of phosphodiestherase type III. enzyme patients who were treated with anagrelide did not get arterial thromboprophylaxis. For those who had already been anticoagulated due to prior major venous TE event we continued venous thromboprophylaxis. Because of the retrospective and” real life” nature of our analysis, involving 14 haematology centres, therapeutic decisions were made by the clinicians, not necessarily according to the same protocoll. Conclusions regarding therapy have been published previously [17].
Statistical analysis
We used Kolmogorov- Smirnov test to determine the
distribution of continuous variables (type I error = 10%). Samples
showing normal distribution were compared with t test for
independent samples (test for variance homogeneity: Levene
test, type I error = 5%). In other cases, the Mann-Whitney U test
with exact probabilities was carried out. Ordinal variables were
also compared using the Mann-Whitney U test. We used Fisher’s
exact test or the exact chi-squared (χ2) test to analyse categorical
variables.
Logistic regression analysis (forward method) was used to
investigate how treatment (anagrelide vs hydroxyurea+aspirin),
age at diagnosis (<60 years vs. >60 years), JAK2V617F positivity (yes or
no), gender (male vs. female) and pre-diagnostic thromboembolism
(yes or no) influenced post-diagnostic TE events.
Since type I error was not adjusted for multiple testing our
results are only descriptive. Statistical analysis was performed
using the open-source R statistical software package, version 3.1.2.
Statistical tests were evaluated at a significance level of 5%.
Ethics and study management
The test was carried out under license from ETT-TUKEB in compliance with the principles of GCP and Helsinki Declaration. Patients gave written consent to use their data anonymously after being informed of the nature of the study.
Result
Patient characteristics and thrombotic risk
We analysed the data of 237 ET patients with an average followup of 10 years (range from 1-29 years). The mean age at diagnosis was 60.9 years. Female predominance was observed with a male: female ratio of 1:2. JAK2V617F mutation positivity was present in 70.5% of the cases. 116 patients received anagrelid therapy, 121 patients were treated with the combination of hydroxiurea and acetylsalcylic acid. Our findings about different treatments, the effect of medication on thrombotic risk, disease progression and survival have been published earlier [17].
Thrombotic events
Figure 1:Occurrence of thromboembolic events before the diagnosis and during follow up.
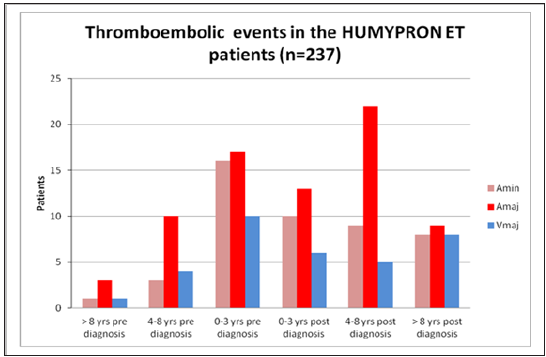
Figure 2:Distribution of thromboembolic events before and after the diagnosis.
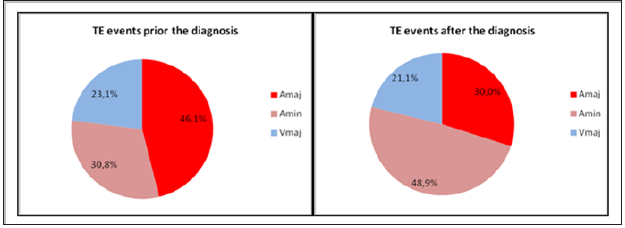
Before the diagnosis 63 patients (26.6%) had 65
thromboembolic events. The earliest TE event occurred 10 years
before diagnosis (Figure 1). Major arterial thrombosis was the most
common complication (n=30, 46.1%). Minor arterial thrombosis
was registered in 20 cases (30.8%), and we found major venous
TE in 15 cases (23.1%). After the diagnosis 76 patients (32.1%)
suffered 90 TE complications. The latest TE episode was registered
19 years after the diagnosis. Minor arterial events being the most
common (n=44, 48.9%). Major arterial TE was observed in 27
(30.0%) and major venous TE in 19 (21.1%) cases (Figure 2).
Minor venous events were not taken into account during the followup
period of 10 years on average. Bleeding complication occured in
case of 8 patients (3.4%).
Those who had thrombotic event prior to ET diagnosis were
significantly more likely to have a thrombotic event after diagnosis
(p <0.001). Prior thrombotic event turned out to be independent
risk factor. At the same time, no significant correlation was found
with any other parameter, either age, diagnostic blood count, or
mutation status (Table 1). Logistic regression analysis (multivariate
model) indicated that only TE prior to diagnosis influence the TE
incidence significantly (p<0.001; Table 2).
Table 1:Characteristics of patients with ET (n= 237) and occurrence of thromboembolic events.
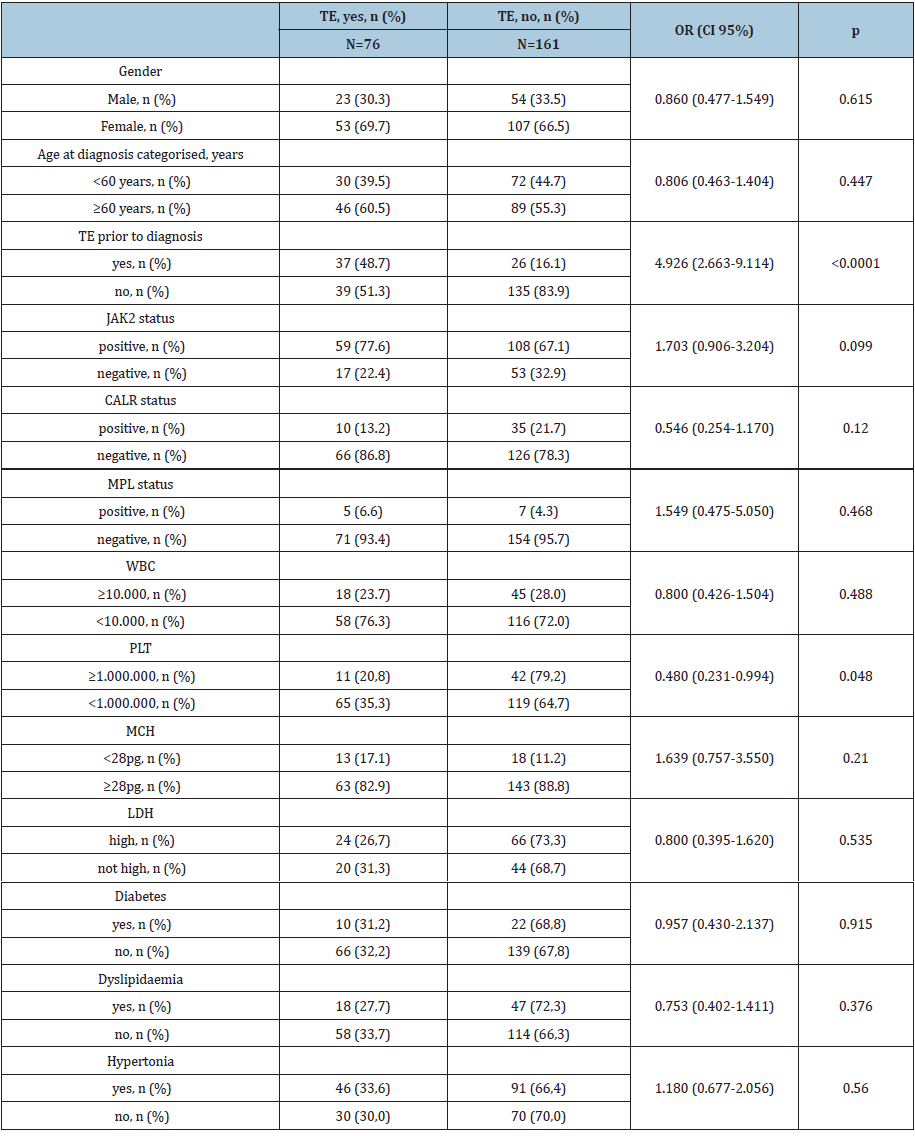
Table 2:Influence of different patient characteristics on post-diagnostic TE complications (total n = 237), logistic regression (multivariate model).

Thrombotic risk stratifications
We applied and analysed Landolfi-, IPSET- and R-IPSET risk stratifications in 237 ET patients of the „HUMYPRON” registry. In Landolfi’s low risk group, 37.5% of patients had a thrombotic event after diagnosis, whereas in the very high-risk group thrombotic events were registered in44.9% of the cases. At the same time the proportion of TE events in Landolfi’s low risk group (37.5%) was higher than what we observed in the medium (23.8%) and high risk (27.8%) groups (Table 3; Figure 3). According to the IPSET thrombosis model 18.8% of the patients in the low-risk group suffered TE complication, while in the high-risk group this proportion was 39.0%. In the intermediate group fewer thrombotic events were registered than in the low-risk group (Table 3; Figure 4).
Table 3:Occurrence of thromboembolic events according to risk categories in Landolfi-, IPSET- and R-IPSET models.
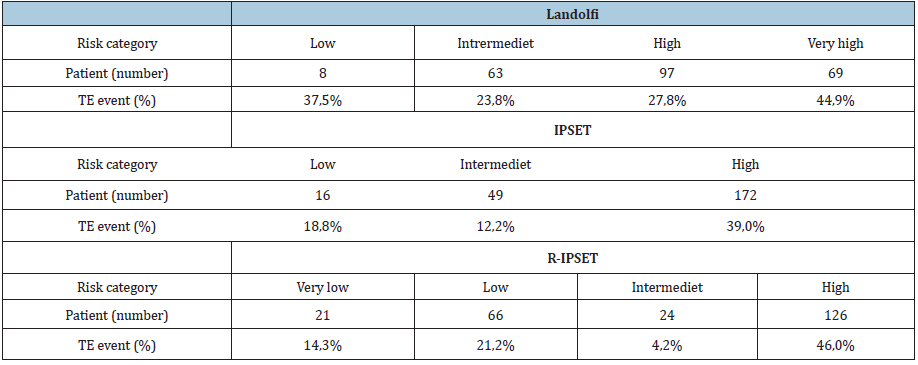
Figure 3:Occurrence of thromboembolic events according to Landolfi thrombosis risk model.
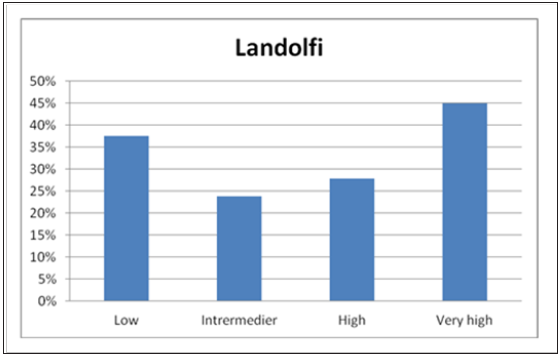
Figure 4:Occurrence of thromboembolic events according to IPSET thrombosis risk model.

Figure 5:Occurrence of thromboembolic events according to R-IPSET thrombosis risk model.
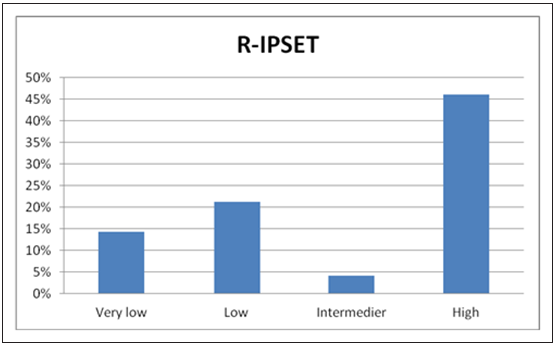
As for the R-IPSET risk stratification, 14.3% of patients of the
very low risk group suffered thrombosis after diagnosis, whereas
this ratio was 46.0% amongst high-risk group patients. In the
intermediate risk group, however, TE events were registered with
lower frequency than in the prior low-, and very low risk categories
(Table 3; Figure 5).
Comparing all categories, it was the R-IPSET high risk group
in which the highest proportion of thrombotic events was
observed after diagnosis (46.0%). Although in all of the three
risk stratification systems it was the highest risk group in which
most TE events occured. (Landolfi: 44,9%, IPSET: 39,0%, R-IPSET:
46,0%). Also, in every system we found significant difference in
the thrombosis-free survival between patients in the highest risk
group and those in the prior risk groups (Landolfi: p=0.032, IPSET:
p<0.001, R-IPSET: p<0.001).
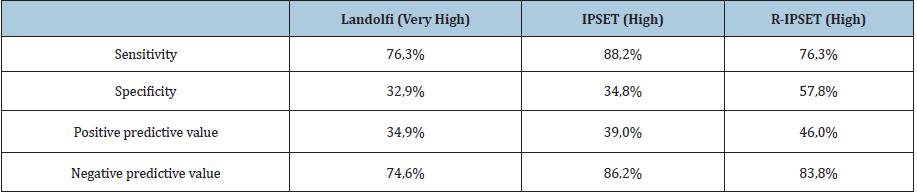
The most sensitive indicator of thrombosis after diagnosis
is the high-risk group of the IPSET system (88.2%). The negative
predictive value is also the highest in the IPSET stratification
(88.2%). As for specificity and positive predictive value the R-IPSET
system turned out to be the best. The Landolfi stratification is a
good theoretical model, but in practice it seemed to be less usable
than the other systems. It also has the disadvantage of using fewer
objective parameters (such as smoking) that are based upon
questioning of the patients. Taking everything into consideration
the R-IPSET system seems more balanced than the others, with the
sum of percentages being the highest. In our study it proved to be
the strongest model (Table 4).
Table 4:Reliability of Landolfi-, IPSET and R-IPSET thrombosis risk models.
CALR positive cases
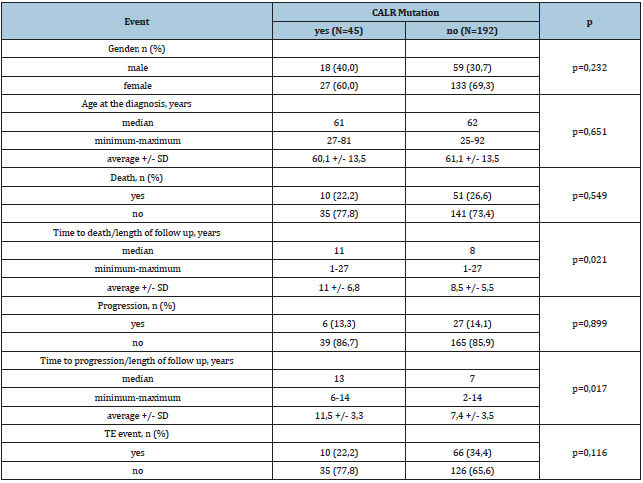
Out of the 237 ET patients CALR mutation was detected in 45 cases. Male dominance and lower median age at diagnosis was observed among CALR patients, but these differences were not significant (Table 5). There was no difference between CALR positive and negative cases regarding the frequency of disease progression and the ratio of fatal outcomes, however, we did find significant difference between the two groups in terms of time to death/disease progression. Patients with CALR mutation had longer progression free- (p=0,017) and overall survival (p=0,021). CALR mutation negative patients suffered TE complications more often (34,4% vs. 22,2%), although the difference was not significant (p=0,116).
Discussion
Thromboembolic complications are the major cause of
morbidity and mortality of ET patients. Because of the favourable
prognosis of the disease the most important aspect of the treatment
is the prevention of TE events. Using thrombosis risk stratification
systems, we can categorize patients into different risk groups and
choose the most appropriate therapeutic option. We analysed
the data of 237 patients from the „HUMYPRON” registry with an
average follow-up period of 10 years. After diagnosis 76 patients
(32.1%) had TE event, while bleeding complication was observed
in 8 cases (3.4%). While before diagnosis the major arterial events
were the most common TE complications, after diagnosis the minor
arterial events occurred most often. Thus, applied therapy seems to
be more protective against major arterial episodes.
When testing Landolfi-, IPSET- and R-IPSET risk stratifications
on our sample collection, we found significant difference in the
thrombosis-free survival of high risk and low risk patients in
all three models (Landolfi: p=0.032, IPSET: p<0.001, R-IPSET:
p<0.001). On the other hand, the incidence of TE events was
lower in the intermediate risk groups than in low-risk groups. The
discrepancy between of our findings and literature data may be due
to the differences in event definition, patient selection and applied
therapy.
R-IPSET system proved to be the strongest model on our sample
set. Landolfi risk stratification turned out to be the least applicable,
which might be explained, along with the differences mentioned
above, with the insufficiency of our data, since it strongly relies on
questioning the patients.
It is known that patients with CALR mutation can expect better
prognosis [18]. A study with a median follow-up of almost 13 years
found that CALR positive cases are associated with younger age,
male dominance and less TE complications [19]. Our results are
similar, though the differences were not significant. However, we
found significant difference in progression free- (p=0,017) and
overall survival (p=0,021) between CALR positive and negative
cases (Table 5).
Table 5:Evaluating the presence of CALR mutation.
Examining potential thrombotic risk factors, we found that only prior thrombotic event shows significant correlation (p<0.001) with TE event after diagnosis. The independent and strongly significant effect of earlier thrombosis on post-diagnostic TE complications anticipates the presence of a prothrombotic phenomenon. Investigations of the last few years revealed that CHIP (clonal haematopoiesis of indeterminate potential) is an independent risk factor of the vascular events. [20,21]. According to literature data the susceptibility of CHIP patients to vascular complications is due to non-driver mutations (ASXL1, TET2, DNMT3A, JAK2) observed in clonal haematopoesis [22-24]. As many of the patients had suffered thrombotic episodes years before the diagnosis it can be presumed that some of the pre-diagnostic TE events may have manifested in the CHIP fase (Figure 1). According to our data we suggest that the TE events observed in ET patients are actually attributes of CHIP and not ET itself. The LCN2 (lypocalin-2) and MMP9 (matrix metalloproteinase-9) produced by neutrophil granulocytes/ monocytes arising from clonal haematopoesis damage the endothel, leading to increased thrombotic risk. [25].
Conclusion
Analyzing data of 237 ET patients of the „HUMYPRON” registry we showed that R-IPSET thrombosis risk system was the most applicable to our sample set. Univariate analysis revealed that thrombotic events before diagnosis have an independent, strongly significant effect on post-diagnostic TE episodes (p<0.001). We suppose that this may be the result of CHIP, and so, patients acquire predisposition to thrombotic complications due to clonal haematopoesis before the actual evolution of ET.
References
- Ma X, Vanasse G, Cartmel B, Wang Y, Selinger HA (2008) Prevalence of polycythemia vera and essential thrombocythemia. American Journal of Hematology 83(5): 359-362.
- Johansson P (2006) Epidemiology of the myeloproliferative disorder’s polycythemia vera and essential thrombocythemia. Seminars in Thrombosis and Hemostasis 32(3): 171-173.
- Cortelazzo S, Viero P, Finazzi GA, D'Emilio A, Rodeghiero F, et al. (1990) Incidence and risk factors for thrombotic complications in a historical cohort of 100 patients with essential thrombocythemia. Journal of Clinical Oncology 8(3): 556-562.
- Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, et al. (2016) The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 127(20): 2391-2405.
- Barbui T, Thiele J, Gisslinger H, Finazzi G, Vannucchi AM, et al. (2016) The 2016 revision of WHO classification of myeloproliferative neoplasms: Clinical and molecular advances. Blood Reviews 30(6): 453-459.
- Tefferi A (2016) Myeloproliferative neoplasms: A decade of discoveries and treatment advances. American Journal of Hematology 91(1): 50-58.
- Tefferi A, Barbui T (2017) Polycythemia vera and essential thrombocythemia: 2017 update on diagnosis, risk‐stratification, and management. American Journal of Hematology 92(1): 94-108.
- Cerquozzi S, Tefferi A (2015) Blast transformation and fibrotic progression in polycythemia vera and essential thrombocythemia: A literature review of incidence and risk factors. Blood Cancer Journal 5(11): e366.
- Cortelazzo S, Viero P, Finazzi GA, D'Emilio A, Rodeghiero F, et al. (1990) Incidence and risk factors for thrombotic complications in a historical cohort of 100 patients with essential thrombocythemia. Journal of Clinical Oncology 8(3): 556-562.
- Jensen MK, de Nully Brown P, Nielsen OJ, Hasselbalch HC (2000) Incidence, clinical features and outcome of essential thrombocythaemia in a well-defined geographical area. European Journal of Haematology 65(2): 132-139.
- Barbui T, Vannucchi AM, Buxhofer-Ausch V, De Stefano V, Betti S, et al. (2015) Practice-relevant revision of IPSET-thrombosis based on 1019 patients with WHO-defined essential thrombocythemia. Blood Cancer J 5(11): e369.
- Landolfi R, Gennaro L (2008) Prevention of thrombosis in polycythemia vera and essential thrombocythemia. Haematologica 93(3): 331-335.
- Tefferi A, Vannucchi AM, Barbui T (2018) Essential thrombocythemia treatment algorithm 2018. Blood Cancer Journal 8(1): 1-6.
- Dombi P, Illés Á, Demeter J, Homor L, Simon Z, et al. (2017) Anagrelide reduces thrombotic risk in essential thrombocythaemia hydroxyurea plus aspirin. European Journal of Haematology 98(2): 106-111.
- Dombi P, Illés Á, Demeter J, Homor L, Simon Z, et al. (2016) Development of the registry for Philadelphia-negative chronic myeloproliferative neoplasia in Hungary. Orvosi Hetilap 157(3): 98-103.
- Gisslinger H, Gotic M, Holowiecki J, Penka M, Thiele J, et al. (2013) Anagrelide compared with hydroxyurea in WHO-classified essential thrombocythemia: The Anahydret Study, a randomized controlled trial. Blood 121(10): 1720-1728.
- Kellner A, Dombi P, Illes A, Demeter J, Homor L, et al. (2020) Anagrelide influences thrombotic risk, and prolongs progression‐free and overall survival in essential thrombocythaemia vs. hydroxyurea plus aspirin. European Journal of Haematology 105(4): 408-418.
- Carla AS, Van Obbergh, Billiet J, Lierman E, Timothy D, et al. (2015) Analysis of phenotype and outcome in essential thrombocythemia with CALR or JAK2 mutations. Haematologica 100(7): 893-897.
- Tefferi, Ayalew EA, Lasho TL, Belachew AA, Ketterling RP, et al. (2014) Calreticulin mutations and long-term survival in essential thrombocythemia. Leukemia 28(12): 2300-2303.
- Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, et al. (2014) Age-related clonal hematopoiesis associated with adverse outcomes. New England Journal of Medicine 371(26): 2488-2498.
- Libby P, Ebert BL (2018) CHIP (clonal hematopoiesis of indeterminate potential) potent and newly recognized contributor to cardiovascular risk. Circulation 138(7): 666-668.
- Jaiswal S, Natarajan P, Silver AJ, Gibson CJ, Bick AG, et al. (2017) Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. New England Journal of Medicine 377(2): 111-121.
- Steensma DP (2018) Clinical consequences of clonal hematopoiesis of indeterminate potential. Hematology 2(22): 3404-3410.
- Buscarlet M, Provost S, Zada YF, Bourgoin V, Mollica L, et al. (2018) Lineage restriction analyses in CHIP indicate myeloid bias for TET2 and multipotent stem cell origin for DNMT3 Blood 132(3): 277-280.
- Rajnics P, Kellner Á, Karádi É, Moizs M, Bödör C, et al. (2016) Increased Lipocalin 2 level may have important role in thrombotic events in patients with polycythemia vera and essential thrombocythemia. Leukemia Research 48: 101-106.
© 2021. Miklos Egyed. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






