- Submissions

Full Text
Novel Approaches in Cancer Study
Identification of Oncogenes in Breast Cancer Development
Pranav Rajashekar1, Chikezie OMadu2 and Yi Lu3*
1Departments of Biology and Advanced Placement Biology, White Station High School, Memphis, Tennessee 28117, USA, pranava1007@gmail.com
2Departments of Biology and Advanced Placement Biology, White Station High School, Memphis, Tennessee 28117, USA, maduco@scsk12.org
3Department of Pathology and Laboratory Medicine, University of Tennessee Health Science Center, Memphis, TN 38163, USA, ylu@uthsc.edu
*Corresponding author: Yi Lu,Department of Pathology and Laboratory Medicine, University of Tennessee Health Science Center, Cancer Research Building, 19 South Manassas Street, Memphis, TN 38163, USA, Email: ylu@uthsc.edu
Submission: September 29, 2020 Published: October 28, 2020

ISSN:2637-773XVolume5 Issue3
Abstract
The role genes play in cancer progression and cancer subtypes has gained interest in research over the years. Numerous studies have focused on the potential proliferation of oncogenes and their transformation from proto-oncogenes. Through various research, these components have led to unique cases of cancer. Oncogenic occurrences are being studied to limit their influences on future generations, as their relation to the increasing rates of specific cancer types has not gone unnoticed. The focus of this will be on the effects of oncogenes and proto-oncogenes in breast cancer development. The specific oncogenes discussed in this paper are sectioned into three groups based on the effects of their mutations, and these three groups are tyrosine-kinase activity, transcription factors, and GTPase activity. Moreover, the study of these oncogenes can reveal a novel understanding of breast cancer, which can then be applied to develop treatments.
Keywords: Oncogene; Proto-oncogene; Tyrosine-kinase activity; Transcription factors; GTPase activity
Introduction
With the growing prevalence of breast cancer cases, the role of oncogenic factors has been demonstrated in many studies. Recently, despite the number of breast cancer cases continuing to increase, growth in the number of cases has slowed down [1]. Studies have consistently shown a relationship between oncogenes and the presence of breast cancer. Numerous oncogenes are related to the proliferation of cells during the stages of breast cancer. These are originally mutated and directly lead to negative effects by inducing overproduction of cells in the body [2]. The deregulation of such genes has been noticed in various cases of breast cancer. Alterations to these genes can cause deformed mechanisms in various cellular functions, such as cell division and growth, and can impede the victim's survival chances [2]. Although there are still many unknowns regarding oncogenes, research is being conducted to understand oncogenes' potential effects on cancer-inducing pathways [1]. Novel understanding regarding the role of oncogenes in breast cancer development as derived from this research is then being used in developing treatments [1]. This paper will focus on certain oncogenes that have facilitated breast cancer development through the alteration of tyrosine-kinase activities, transcription factors, and GTPase activities. Recent advances in breast cancer development and treatment will also be discussed [3-6].
Tyrosine Kinase-Affecting Breast Cancer Oncogenes
As part of the Epidermal Growth Factor Receptor (EGFR) family, the EGFR gene, also commonly known as ErbB1, codes for the EGFR and is located on chromosome-7 [7-9]. EGFR regulates certain cellular processes, such as cell growth and proliferation. It does so through a receptor tyrosine pathway, a pathway in which the receptor is dimerized then phosphorylated [9]. Specifically, the binding of ligands to the receptors induces the dimerization of EGFR with other receptors. This occasionally creates a heterodimer group [9]. In the following events, the cytoplasmic component of the receptor undergoes a structural change, which allows for phosphorylation when the tyrosine kinase pathway is induced [8]. For accurate cellular growth regulation throughout the cell cycle, numerous biological pathways depend on this Receptor Kinase Pathway (RTK) to be induced through mediums like the EGFR proto-oncogene. However, mutations can act onto these proto-oncogenes and turn them into oncogenes that promote tumorigenesis. The amplified version of the gene leads to the overexpression of the EGFR genes, causing an increase in the number of EGFR within the cell [8]. Elevated amounts of EGFR can then cause an increase in the induction of genes throughout the cell. The Ras protein can also come into play through the tyrosine kinase process, which causes an increase in cell proliferation [8]. Also, the tyrosine kinase process plays a role in the transition from the G1 and S phases of the cell cycle, critical for cell survival and growth [8]. The erbB2 gene's location is along chromosome-17, and it functions as a transmembrane tyrosine receptor [4]. In addition to this, the erbB2 gene can encode a product that can act as a transmembrane glycoprotein, which allows for the emergence of the EGFR family [3]. This family consists of four receptors, where the ErbB2 is primarily targeted as the preferred binding site since it lacks ligand binding specificity [6]. The overexpression of the gene can cause the ErbB2 receptor to increase in cells drastically, and growth factor stimuli can be reached to give rise to malignant tumors [3]. The erbB2 gene is found to be overexpressed in approximately 30% of breast cancer cases, making it a significant cause for the malignancy [4]. Also known as the HER2 gene, the erbB2 oncogene codes for a receptor that always has an open structure, further making the receptor the primary target of ligands. Overexpression of the HER2 gene causes the HER2 receptors to increase throughout the cell, which results in an expanding rate of cell growth and multiplication. After the dimerization process that accompanies such tyrosine receptors, the termination of a cell cycle inhibitor called p27Kip1 is induced, causing the cell cycle to progress at increased rates [3,5]. This increase leads to a proliferation of cells, which then develop into tumors responsible for breast cancer [10-12].
Another proto-oncogene, called the SRC gene, influences cellular growth and tumorigenesis in humans, as it codes for a nonreceptor tyrosine kinase that influences the induction of breast cancer. Prominently used in cell signaling, the SRC gene can lead to further growth and expansion in human cells if not regulated. The SRC gene consists of Tyr416 and Tyr527, which are both respective for autophosphorylation-promoting and phosphorylation-inhibiting sites [13]. Furthermore, it contains an SH3 domain, SH2 domain, tyrosine kinase site, and a C-terminal regulatory site [13]. In numerous people with breast cancer, an increase in the SRC gene expression and SRC Family Kinases (SFK) activity is present. The levels of SFK or SRC family kinases are relatively higher in tumorigenesis-inducing tissues than normal tissues in mammals [10]. Studies show that the dephosphorylation of the location Tyr530 on the SRC gene could have led to these increased kinase activity levels and normal levels of SRC proteins [10]. SFK induction could lead to cancer because specific SFKs amplify signals that lead to cell proliferation, growth, and survival [10,12]. If the SFK activity increases in the cell exceeding that of normal cells, increased tumorigenic rates can be induced in breast cancer patients. Furthermore, the effects of SFK can carry over to phosphorylating Receptor Tyrosine Kinases (RTKs) at numerous Tyr-like locations, and this can change their signaling mechanisms and product over time [11,12]. In certain mice with the HER2 gene, the activation and induction of the SRC gene correlated with the tumorigenesis in the mice, which leaned toward the potential dependence of SRC genes on the EGFR family during the progression of breast cancer risks in mammals [11].
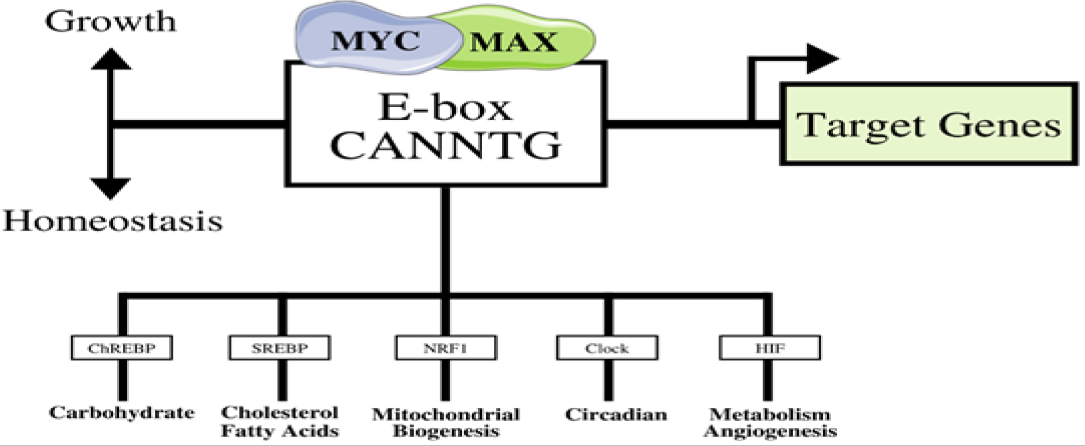
Figure 1: The MYC-MAX complex that binds to the E-Box. Triggered by the MYC gene’s overexpression, other transcription factors are overregulated in response, as they are usually regulated by homeostasis. The results lead to cellular growth (adapted from [61]).
Transcription Factor-Affecting Breast Cancer Oncogenes
Identified thirty years ago, the c-MYC gene continues to play a prominent role in breast cancer development. The proto-oncogene is regulated for normal cellular maturity, cellular proliferation, and is essential for continuing the cell cycle [14-16]. Since the c-MYC gene's oncogenic component leads to unregulated and increased growth of cells, breast cancer has been linked to this gene through numerous biological pathways [14,15]. The gene is located on chromosome 8, and it is commonly translated to produce the MYC protein, which acts as a transcription factor [14,15]. The protein has a shape similar to a bHLH (basic helix-loop-helix) protein, and it binds to E-boxes along specific sequences with compliance of an X-factor; as shown in Figure 1, the MYC-MAX is a crucial protein complex that affects the activation of other transcription factors, leading to cellular growth [14,17]. Moreover, the MYC protein directly influences cell cycle progression from the G1 developmental phase to the S phase by activating certain molecules like Cdk4, cyclin D1, E2F1, and more [14]. By promoting the phase changes in the cell cycle, an increase in the number of MYC proteins can have a negative impact by increasing cell proliferation rates as well, causing tumors to form. Aside from behaving as a transcription factor, the MYC protein has other effects. Telomerase has been a primary target for the MYC protein, as the production of TERT (Telomerase Reverse Transcriptase) induces the activation of telomerase-producing processes [18]. Furthermore, telomerase provides DNA sequences that increase cell longevity and prolong its presence. The levels of TERT have been relatively high compared to other proteins in numerous breast cancer cases [14,18]. The oncogene also promotes the development of new blood vessels, or angiogenesis, to support the growth of tumors in humans [14]. By increasing the levels of miR9, a regulating agent that prevents transcription from occurring, the suppressor in metastasis, E-cadherin, is reduced, promoting the growth of breast cancer in humans [14]. As highlighted above, the c-MYC gene has transcriptional value in regulating tumor development in a range of breast cancer cases.
The TP53 gene is found to be altered in approximately 20-40% of all breast cancer cases in 2003 [19]. This gene is essential for the negative feedback present in cell proliferation. In essence, the gene produces a tumor-suppressor protein and transcriptional factor that decreases the rate of tumorigenesis. The TP53 gene is located on chromosome-17 and produces p53, a crucial protein for DNA binding, transcription of other proteins, and regular cellular growth [20-22]. If the TP53 gene is mutated, the cell cycle can be disrupted, and cells in the body can potentially divide too quickly, leading to the development of various cancer types. The p53 protein is shaped with a structure of beta-sheets sequenced together in an antiparallel fashion [21]. This composition gives the protein its flexibility when binding to DNA as a transcription factor [21]. To regulate the transcription of other sequences, the p53 protein binds at specific locations along the introns or promoter of the gene region [21]. However, the spaces between each binding location within the sequence vary, hinting that certain signaling mechanisms are used to activate genes through p53 mediums [21]. By binding to these various sites on target genes, p53 can facilitate the transcription of proteins that regulate mechanisms needed for cell survival. For instance, Puma and Hdm2 are examples of proteins synthesized after the activation of transcription by the p53 protein, and both proteins respectively influence apoptosis and p53 control within the cell [23]. On the other hand, mutations can change the TP53 gene, resulting in conformational and functional changes to the p53 protein. Even though UV light exposure and other carcinogens affect the TP53 gene's function, most are missense mutations, where a different protein can be formed with a single base change in the DNA sequence [21,23]. With this, the shape of the p53 protein can be changed as internal interstices can be formed within the protein [21]. These unnecessary structural changes can induce inappropriate binding to target DNA sites. The transcriptional processes that are activated to produce proteins for cellular regulation are terminated due to these mutations. Furthermore, many biological pathways, such as apoptosis, can no longer regulate cell growth, and as a result, proliferation rates will rise. This is evident in numerous breast cancer cases, as tumorigenesis has been highly influenced by the p53 protein's absence and disruption of the TP53 gene [20].
The GATA family consists of various genes like the GATA3 gene that code for zinc-finger transcription factors, known for an additional zinc component [24-27]. These proteins attach to specific DNA segments that induce protein production for internal processes in the human cell; therefore, the GATA3 gene's deregulation can allow tumorigenesis to occur. The GATA3 gene is located on chromosome-10 of humans [24]. Additionally, it produces the GATA binding protein 3, which acts as a transcription factor to activate other proteins and pathways, such as mammary development in luminal breast epithelial cells [25]. One pathway that is induced through a positive feedback loop is the estrogen receptor alpha (ER-alpha) signaling pathway and its relationship with the expression of the GATA3 gene [28,29]. Through this feedback loop, the GATA3 gene produces a protein that binds to cis-regulatory components of the estrogen receptor alpha gene [29]. As a transcription factor, the GATA3 protein binding is essential for RNA Polymerase II to bind to the target gene's promoter region [29]. Through this, estradiol can bind to receptors, causing cell cycle progression and potentially resulting in humans' cellular growth. When a frameshift mutation occurs, the GATA3 protein undergoes a cycle and becomes overexpressed [28]. This causes ER-alpha receptors and GATA3 proteins to be translated at higher rates, leading to increased cell proliferation levels. More research is being done on how estrogen receptor alpha mutations cause breast tumors to develop in recent experiments. It has only recently been added to genetic breast cancer risks. Human breast cancer cases have seen a correlation between increased GATA3 expression and ER-positive tumors, as ER-alpha feedback loops are positive concerning GATA3 regulation [26]. Accelerated expression of the GATA3 gene led to increased growth in mammary glands, as the same proportions have been observed in human cases as well [26]. The deregulation of the GATA3 gene can cause potential overexpression in humans as its relationship with ER-alpha can influence breast tumors to develop at accelerated rates.
GTPase-Affecting Breast Cancer Oncogenes
The Rab1a gene, which primarily influences protein transport in the endomembrane system, is crucial for the induction of breast cancer through GTPase functions [30]. Located on chromosome 2, the Rab1a gene is most known for its placement among the Ras gene family, which uses cellular signaling to promote human cells' growth and development [30]. A pathway affected by the Rab1a gene is the mammalian target of rapamycin (mTOR) signaling. In this process, the endoplasmic reticulum & Golgi apparatus have the task of protein transport via vesicles [31]. To accomplish this, chemical signaling needs to be promoted between the two organelles. Through this nutrient signaling, the cell can promote growth and protein specialization through the interaction between mTOR pathways and Rab1a proteins [31]. Specifically, a factor called AA, which is used to stimulate mTORC1 in the nutrient signaling, increases based on the expression levels of the Rab1a gene [31,32]. AA is prominent in cellular growth and in the Golgi apparatus's function, as the stimulation of GTP-dependent pathways between Rab1a and mTORC1 is in the organelle itself [31]. When mutations occur, the oncogenic version of the Rab1a gene stimulates an increase in mTORC1 signaling as well. This can stem from the increasing levels of the AA factor resulting from overexpression of the Rab1a gene [31,33]. Numerous cases have reported an increase in breast cancer metastasis and tumor growth from the overexpression of the oncogene [31]. Overall, the increase in the interaction between mTORC1 signaling and Rab1a gene expression can lead to cell expansion and invasiveness in humans. This can lead to increased rates of tumorigenesis, leading to eventual proliferation into breast cancer.
Another GTPase protein that is produced derives from the Rac1 gene. This gene belongs to a subclass called Rho genes, which are components of the superclass Ras genes that code for GTP-binding proteins [34-38]. The Rac1 gene is located on chromosome 7. Like other functional Rho GTPases, the protein is constantly cycling through activated and deactivated forms through the roles of regulators, including GEF, GAP, and GDI [34,35]. GEF is commonly associated with permitting the GTP-bound form to develop, but the GAP is primarily involved in the inactivation of the GTPase [37]. The expression of the Rac1 gene and GEF and GAP effects can induce cell growth and motility through Rho GTPase signaling [36]. Moreover, these regulators are present at various levels during the progression of certain cancers within humans [36]. The increased regulation of the Rac1 gene can cause changes in upstream signaling, another way that leads to pathways for cellular growth and tumorigenesis [35]. GEF-related upregulation has been closely studied from the past years and increases in GEF levels have introduced increased cell growth and gene expression in cells. For instance, the P-Rex1 is a GEF that is specific to the Rac1 gene itself [36]. Overexpression of P-Rex1 has shown that increases in the use of tyrosine-kinase and other G-protein receptors have been integrated into pathways of cellular growth and tumor promotion [36]. Another GEF that influences the increasing risk of breast cancer in humans is Ect2 [38]. In proto-oncogene-containing cells, Ect2 diffuses and binds to microtubules, promoting normal cytokinesis in the cell; however, the cell's oncogenic version induces Ect2 to promote overexpression of the gene, causing the MEK/Erk pathway to be overexpressed [36]. The overexpression of this pathway can result in the mis regulation of certain processes, thereby promoting cancer risks. A splice variant of the Rac1 gene, Rac1b, is also related to the induction of breast cancer risks in humans [35]. Overexpression of this gene sub-type induces the increasing levels of Reactive Oxygen Species (ROS), which can have mutations leading to cancer cells' growth in humans. This leads to an increase in the Snail transcription factor, leading to mammary cell growth and EMT, which is conducive to cell invasion and increased development of tumors [35]. Rho GTPases such as the Rac1 gene can have significant effects on the progression of breast cancer.
Concerning the GTPases interconnected in pathways to promote breast cancer, the Arf6 gene is another potential oncogene that has been researched in the past and is known to lead to cell invasiveness and tumorigenesis in breast cancer patients. The ADP-ribosylation factor family, commonly known as the Arf family, is a group of GTPases that belong to the superfamily of Ras factors. These GTPases bind to G domains to promote cell signaling and activation of certain tasks. The Arf6 gene is a component of the Arf family that produces a specific G-binding protein, and it is located on chromosome-14 [39]. Like the Rac1 gene and Rho GTPases, the GEF and GAP factors are essential for the functioning of the chemical signaling that results from the GTPase [40]. Besides, the GEFs tend to activate the complexes, but the GAPs are primarily localized with the inactivation of the proteins [37]. Ultimately, the Arf6 can also become an oncogene, as it influences pathways that lead to cell migration and malignant tumorigenesis in humans. For cell migration to occur, cancerous cells need to detach from tumors and migrate through the extracellular matrix (ECM) and adhere to certain locations [40,41]. This can cause cells to proliferate in different locations while contributing to the growth of tumors and breast cancer in general. Overexpression of the Arf6 gene and specific GEFs are showcased to connect with this defective cell phenotype. In recent studies, the GEF BRAG2 and the overexpression of Arf6 have resulted in this invasive behavior of cells when paired with the expression of the EGF genes [40,42]. In this process, E-cadherin's function, which has a large component in Adheren Junctions (AJs) and is needed in cell-adhesion mechanisms, is defected [40]. The Arf6 gene's function is also related to that of the Rac1 gene since the activation of the Rac1 gene can be induced with its fashions in promoting breast cancer [40].
Treatments and Advancements
In clinical research, the c-MYC gene has been observed to be overamplified in many breast cancers cases, including the aggressive subtype called triple-negative breast cancer [43]. During gene amplification and increased cell growth, it has been postulated that gene blockade can induce disruption of the ongoing oncogenic processes [44]. Moreover, scientists have found the inactivation of the MYC gene to lead to suppressed tumorigenesis [43,44]. However, this has only occurred in preclinical trials and has yet to be researched further. In addition to this development, recently, a molecule that degrades the MYC oncogene through the uses of proteases has been found [43]. The molecule is called C1572, and it specifically correlates with the degradation of cancer stem cells in triple-negative breast cancer models that utilize xenograft tissues. It may thus be used in future clinical studies [43].
Research has also been done to treat breast cancer by targeting the TP53 gene. This gene codes for a transcription factor called p53, and the absence of this protein can induce more severe pathways leading to increased tumorigenesis. For instance, the drug Paclitaxel has portrayed a significant relation with the absence of the p53 protein [45,6]. The loss of p53 was related to increased levels of apoptosis induced by Paclitaxel and independent of the p53 gene [45,46]. This causes cells to stop proliferating at high rates since mitosis is disturbed by the loss of the p53 function [45]. The p53 gene can also provide insight towards molecular markers that showcase tumor response in systems. Treatment-resistant tumors can be developed through radiotherapy since a loss of p53 function in tumor cells can cause apoptotic mechanisms to be disturbed [45]. This allows scientists to connect the function of p53 to the growth of tumors in future studies regarding radiotherapy [45].
Although the GATA3 gene's uses have not shown much definite success, the role of the GATA3 gene in breast cancer development is still being studied. Currently, experiments are being done to find biological markers to target the gene and prevent cancer growth. Studies in mice have shown that breast cancer prognosis is evident with changes in GATA3 gene levels in relation to p18 gene deficiency [47]. According to a study, the loss of the p18 gene and high GATA3 levels have been related to the development of ER-positive luminal tumorigenesis [47]. Furthermore, the promotion of the gene has shown differentiation in luminal cells [47]. Another area studied is the expression of E-cadherin and how it can interact with varying levels of MCF-7 and MDA-MB-231 cells to promote the reversal of Epithelial-Mesenchymal Transition (EMT) [48]. This can prevent the migratory phenotype of breast cancer [48]. The evidence provided by these ongoing experiments shows that the GATA3 gene must continue to be studied extensively to reduce breast cancer risks.
For tyrosine kinase-based genes, such as the gene that codes for the HER2 receptor, many treatments are based on inhibiting these kinases. Trastuzumab and Pertuzumab are antibodies used in breast cancer treatment. In clinical trials, the addition of kinase inhibitors enhanced the effects of the treatments. In an experiment conducted by Canonici et al. [49] tyrosine kinase inhibitors (TKIs), afatinib, lapatinib, and neratinib, were used with Trastuzumab and Pertuzumab to test cell proliferation effects in HER2 breast cancer cells [49]. Pertuzumab by itself did not inhibit cellular growth in breast cancer tumors, but when lapatinib was added with Pertuzumab, the responses towards Trastuzumab were enhanced [49]. Previous studies also hint that neratinib is useful in targeting tyrosine kinases in the early onset of breast cancer [50]. From these, it is understood that further research is needed to find more inhibiting factors to reduce breast cancer risks in humans.
Treatments are also being researched for limiting the cell proliferation from the overexpression of the EGFR gene. This gene also interacts with tyrosine kinase mechanisms, which evinces the uses of inhibitors to blockade its functions as an oncogene. However, little success has been achieved in clinical trials and research with this gene [51]. In the past, lung carcinomas, like NSCLC, have been portrayed as cancers that grow on major mechanisms related to the EGFR gene [51]. Since this is not the only path towards cell proliferation in breast cancer cases, many EGFR mutations can still occur, but the results of EGFR gene disruption can be small enough for cancer to still progress [51]. In contrast to this clinical approach, one treatment has proven successful in triple-negative breast cancers. Cetuximab is a developed monoclonal antibody that acts as an inhibitor on the gene's downstream pathway [52]. Through this, cell growth and proliferation can be stunted in oncogenic versions of the EGFR gene [52].
The SRC gene has also been researched as a drug target. Many drugs have been synthesized to disrupt the tyrosine kinase activity that is overexpressed along with gene amplification. For instance, the Dasatinib treatment (TKI) was met with an unsuccessful trial when alone, but a modification occurred to increase anti-cancer phenotypes [53,54]. Even though cell migration was decreased with the addition of the TKI, the combination of Dasatinib, Cetuximab, and Cisplatin was implemented [53]. This combination induced apoptosis and the inhibition of EGFR and MAPK pathways, thereby decreasing cell proliferation [53]. The cell migration rate was also decreased in response to the three-compound drug associated with the trial [53]. Also, another TKI that is being investigated is Ponatinib, which is used to inhibit kinases with the SRC gene and inhibit growth factor-inducing mechanisms [54,55]. This can limit the cellular growth rate as well as tumorigenesis rates [55].
As part of the superfamily of GTPases, the Rac1 gene is currently being researched for specific molecular targets that can help decrease cell migration and growth in breast cancer cases. A few inhibitors have been used to test the efficacy of decreasing the oncogenic phenotypes of the products. For instance, NSC23766 is a chemical compound developed by scientists to inhibit the Rac1 gene's role as a GTPase [56]. This molecule prevents the activation of Rac1 GEFs, such as TRIO and TIAM1, in this case. It is thus useful in inducing characteristics that decrease metastasis and growth of tumors [56]. With breast cancer, the use of NSC23766 induces apoptosis and limits cellular growth in breast cancer cells while not affecting standard mammary cells [56]. The role of Rac1 in radiation therapy is also being explored. In the experiment conducted by AL Hein et al. [57] the HFR-cancer cells were selected since the levels of the Rac1 gene increased by radiation therapy [57]. Moreover, the oncogene's inhibition with molecule N17Rac1 decreases HFR-breast cancer cell survival rates [57]. Through this, radiation therapy can have significant effects while ensuring that cancer cells do not develop resistance.
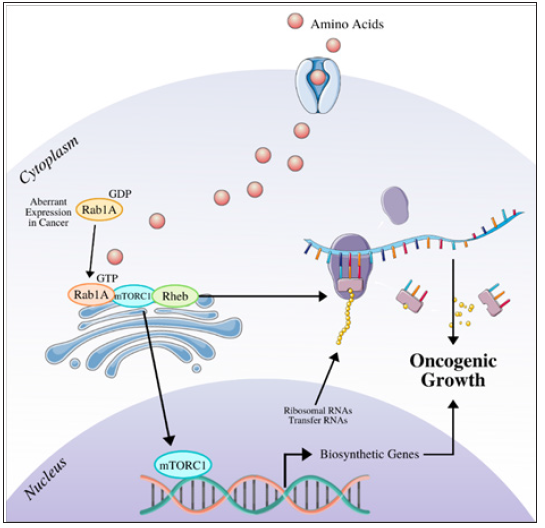
Research is still being conducted to find potential pathways to inhibit cancer growth in humans through the Arf6 gene medium. As an oncogene that acts as a GTPase, numerous mechanisms are activated that promote cell migration and proliferation [58]. Therefore, inhibiting these features could also impede tumorigenesis in breast cancer cases. SGC-7901 cells were considered for a study by Junlan Qiu et al. [58] with a group of cells growing in standard conditions with Arf6 oncogene eradicated and nonfunctional. The experiment's result was that SGC-7901 cells without Arf6 had significantly less cell proliferation and migration of cancer cells, which decreased the probability of tumorigenesis and cell growth [58]. Even though this process was done in vitro, it can be deduced that future implementation of the Arf6 deletion can lead to fewer breast cancer risks. However, more research is being conducted to understand the gene's uses in treatments for breast cancer. Specific to the Rab1a gene, the mTOR pathway is made up of mTOR1 and mTOR2 [59]. As shown in Figure 2, the pathway is prominent in the oncogenic response with the Rab1a gene, and it increases cellular growth and promotes cell proliferation. This is a closely researched topic in preventing the risks that accompany breast cancer in humans. The mTOR1 subunit is activated by a molecule called p-P70S6K [60]. When the Rab1a gene experiences overexpression in breast cancer cases, there has also been the case of an increase in p-P70S6K, leading to cell growth from mTOR signaling [59]. In an experiment conducted by Hui et al. [59] researchers deregulated the expression of the Rab1a gene, which suppressed the proteins produced from p-P70S6K; consequently, cell proliferation was significantly decreased, and tumorigenesis did not progress [60]. Another experiment in vitro was done to explore the effects of the Rab1a gene expression. A siRNA unit was added with the gene, while another group acted as a control. In the experimental group, the deregulation of the Rab1a expression was noticed in breast cancer cell lines MDA-MB-231 and BT-549 [59]. It was then concluded that the number of cells migrating decreased when siRNA was added to the Rab1a gene [59]. Advances made in combating cell invasion, such as those described above, will significantly improve treating metastatic cancer [60-62]
Figure 2: The mTOR signaling that is induced by increasing levels of Rab1a expression. The result is increased protein production and tumorigenesis (adapted from [62]).
Discussion
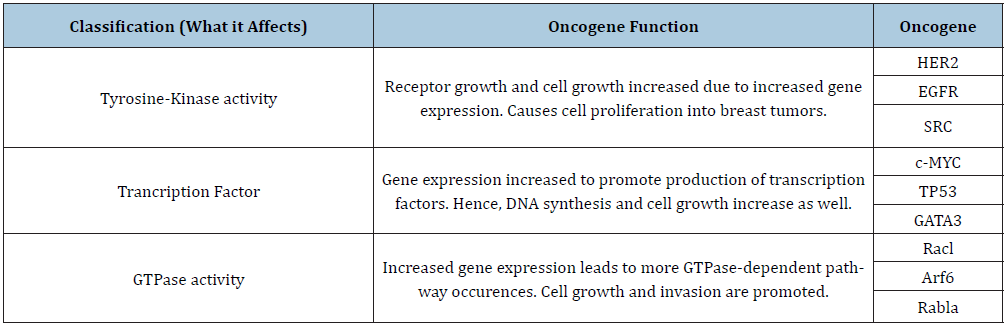
By reviewing the potential effects of the previously identified oncogenes, it is clear that they hold a prominent role in breast cancer development. Tyrosine-kinase activity, transcription factors, and GTPase activity are all targeted oncogenes pathways to affect accelerated cell growth and migration. With regulation methods impeded and growth unchecked, breast cancer cases are currently being researched to reduce the disease's effects in future patients. Table 1 shows an overview of the oncogenic classifications and examples discussed in this paper regarding breast cancer induction. Despite the research done in the past, there is still more to learn about oncogenes and their cancer development role. Furthermore, the treatments found are still being researched. Given time, successful clinical trials and further research can be implemented to test these drugs and methods so that breast cancer risks can be reduced in the future.
Table 1: An overview of the oncogenes and their classifications. Along with this, the main function that leads to breast tumor growth is also listed.
References
- Suter R, Marcum JA (2007) The molecular genetics of breast cancer and targeted therapy. Biologics 1: 241-258.
- Lee EY, Muller WJ (2010) Oncogenes and tumor suppressor genes. Cold Spring Harb Perspect Biol 2: a003236.
- Tan M, Yu D (1970) Molecular mechanisms of ErbB2-mediated breast cancer chemoresistance. Adv Exp Med Biol 608: 119-129.
- Bertucci F, Borie N, Ginestier C, Groulet A, Jauffret EC, et al. (2004) Identification and validation of an ERBB2 gene expression signature in breast cancers. Oncogene 23: 2564-2575.
- Le X, Claret F, Lammayot A, Tian L, Deshpande D (2003) The role of cyclin-dependent kinase inhibitor p27Kip1 in anti-HER2 antibody-induced G1 cell cycle arrest and tumor growth inhibition. J Biol Chem 278: 23441-23450.
- Wieduwilt MJ, Moasser MM (2008) The epidermal growth factor receptor family: Biology driving targeted therapeutics. Cell Mol Life Sci 65: 1566-1584.
- Masuda H, Zhang D, Bartholomeusz C, Doihara H, Hortobagyi GN, et al. (2012) Role of epidermal growth factor receptor in breast cancer. Breast Cancer Res Treat 136: 331-345.
- Voldborg BR, Damstrup L, Thomsen SM (1997) Epidermal growth factor receptor (EGFR) and EGFR mutations, function and possible role in clinical trials. Annals of Oncology 8: P1197-P1206.
- Bhargava R, Gerald WL, Li AR, Pan Q, Lal P, et al. (2005) EGFR gene amplification in breast cancer: Correlation with epidermal growth factor receptor mRNA and protein expression and HER-2 status and absence of EGFR-activating mutations. Mod Pathol 18: 1027-1033.
- Irby RB, Yeatman TJ (2000) Role of Src expression and activation in human cancer. Oncogene 19: 5636-5642.
- Wheeler DL, Iida M, Dunn EF (2009) The role of Src in solid tumors. Oncologist 14: 667-678.
- Sirvent A, Benistant C, Roche S (2012) Oncogenic signaling by tyrosine kinases of the SRC family in advanced colorectal cancer. Am J Cancer Res 2: 357-371.
- Roskoski R (2004) Src protein-tyrosine kinase structure and regulation. Biochem Biophys Res Commun 324: 1155-1164.
- Xu J, Chen Y, Olopade OI (2010) MYC and breast cancer. Genes Cancer 1: 629-640.
- (2020) MYC MYC proto-oncogene, bHLH transcription factor [Homo sapiens (human)]- Gene.
- Fallah Y, Brundage J, Allegakoen P, Haq ANS (2017) MYC-driven pathways in breast cancer subtypes. Biomolecules 7: 53.
- Chen Y, Olopade OI (2008) MYC in breast tumor progression. Expert Rev Anticancer Ther 8: 1689-1698.
- Hines WC, Fajardo AM, Joste NE, Bisoffi M, Griffith JK, et al. (2005) Quantitative and spatial measurements of telomerase reverse transcriptase expression within normal and malignant human breast tissues. Mol Cancer Res 3: 503-509.
- Dale BA (2003) TP53 and breast cancer. Hum Mutat 21: 292-300.
- Eeles RA, Bartkova J, Lane DP, Bartek J (1993) The role of TP53 in breast cancer development. Cancer Surv 18: 57-75.
- Olivier M, Hollstein M, Hainaut P (2010) TP53 mutations in human cancers: Origins, consequences, and clinical use. Cold Spring Harb Perspect Biol 2: a001008.
- (2019) TP53 tumor protein p53 [ Homo sapiens (human)] - Gene.
- Beckerman R, Prives C (2010) Transcriptional regulation by p53. Cold Spring Harb Perspect Biol 2: a000935.
- (2020) GATA3 GATA binding protein 3 [Homo sapiens (human)] - Gene.
- Bhargava R, Esposito N, Dabbs D (2011) Immunohistology of the breast. Diagnostic Immunohistochemistry, Pp. 763-819.
- Emmanuel N, Lofgren KA, Peterson EA (2018) Mutant GATA3 actively promotes the growth of normal and malignant mammary cells. Anticancer Res 38: 4435-4441.
- Takaku M, Grimm SA, Roberts JD, Chrysovergis K, Bennett BD, et al. (2018) GATA3 zinc finger 2 mutations reprogram the breast cancer transcriptional network. Nat Commun 9: 1059.
- Gustin JP, Miller J, Farag M, Rosen DM, Thomas M, et al. (2017) GATA3 frameshift mutation promotes tumor growth in human luminal breast cancer cells and induces transcriptional changes seen in primary GATA3 mutant breast cancers. Oncotarget 8: 103415-103427.
- Eeckhoute J, Keeton EK, Lupien M, Krum SA, Carroll JS, et al. (2007) Positive cross-regulatory loop ties GATA-3 to estrogen receptor alpha expression in breast cancer. Cancer Res 67: 6477-6483.
- (2020) RAB1A RAB1A, member RAS oncogene family [Homo sapiens (human)] - Gene.
- Yang X, Li X, Zhang Y, Rodriguez L, Xiang MQ, et al. (2016) Rab1 in cell signaling, cancer and other diseases. Oncogene 35(44): 5699-5704.
- Xu B, Li X, Yang Y, Mei YZ, Hui LR, et al. (2015) Aberrant amino acid signaling promotes growth and metastasis of hepatocellular carcinomas through Rab1A-dependent activation of mTORC1 by Rab1A. Oncotarget 6(25): 20813-20828.
- Thomas JD, Zhang Y, Wei Y, Hung C, Lauea EM, et al. (2014) Rab1A is an mTORC1 activator and a colorectal oncogene. Cancer Cell 26(5): 754-769.
- (2020) RAC1 Rac family small GTPase 1 [Homo sapiens (human)] - Gene.
- Haga RB, Ridley AJ (2016) Rho GTPases: Regulation and roles in cancer cell biology. Small GTPases 7(4): 207-221.
- Kazanietz MG, Caloca MJ (2017) The Rac GTPase in cancer: From old concepts to new paradigms. Cancer Res 77(20): 5445-5451.
- Schnelzer A, Prechtel D, Knaus U, Dehne K, Gerhard M, et al. (2000) Rac1 in human breast cancer: Overexpression, mutation analysis, and characterization of a new isoform, Rac1b. Oncogene 19(26): 3013-3020.
- Porter A, Papaioannou A, Malliri A (2016) Deregulation of Rho GTPases in cancer. Small GTPases 7(3): 123-138.
- (2020) ARF6 ADP ribosylation factor 6 [Homo sapiens (human)] - Gene.
- Casalou C, Faustino A, Barral DC (2016) Arf proteins in cancer cell migration. Small GTPases 7(4):270-282.
- Li R, Peng C, Zhang X, Yuewei W, Shida P, et al. (2017) Roles of Arf6 in cancer cell invasion, metastasis and proliferation. Life Sci 182: 80-84.
- Morishige M, Hashimoto S, Ogawa E, Yoshinobu T, Hirokazu K, et al. (2008) GEP100 links epidermal growth factor receptor signalling to Arf6 activation to induce breast cancer invasion. Nat Cell Biol 10(1): 85-92.
- Klauber-DeMore N, Schulte BA, Wang GY (2018) Targeting MYC for triple-negative breast cancer treatment. Oncoscience 5(5-6): 120-121.
- Chen H, Liu H, Qing G (2018) Targeting oncogenic Myc as a strategy for cancer treatment. Signal Transduct Target Ther 3: 5.
- Varna M, Bousquet G, Plassa LF, Bertheau P, Anne J (2011) TP53 status and response to treatment in breast cancers. J Biomed Biotechnol 2011: 284584.
- Wahl AF, Donaldson KL, Faircnild C, Lee FY, Foster SA, et al. (1996) Loss of normal p53 function confers sensitization to Taxol by increasing G2/M arrest and apoptosis. Nat Med 2(1): 72-79.
- McCleskey C, Penedo B, Thuy L, Kui Z, Omar H, et al. (2015) GATA3 expression in advanced breast cancer: Prognostic value and organ-specific relapse. Am J Clin Pathol 144(5): 756-763.
- Yan W, Cao QJ, Arenas RB, Brooke B, Rong S (2010) GATA3 inhibits breast cancer metastasis through the reversal of epithelial-mesenchymal transition. J Biol Chem 285(18): 14042-14051.
- Canonici A, Ivers L, Conlon NT, kasper P, Nicola G, et al. (2019) HER-targeted tyrosine kinase inhibitors enhance response to trastuzumab and pertuzumab in HER2-positive breast cancer. Invest New Drugs 37(3): 441-451.
- Dhillon S (2019) Neratinib in early-stage breast cancer: A profile of its use in the EU. Clin Drug Investig 39(2): 221-229.
- Nakai K, Hung M, Yamaguchi H (2016) A perspective on anti-EGFR therapies targeting triple-negative breast cancer. Am J Cancer Res 6(8): 1609-1623.
- Sabatier R, Lopez M, Guille A, Emilien B, Nadine C, et al. (2019) High response to cetuximab in a patient with EGFR-amplified heavily pretreated metastatic triple-negative breast cancer. JCO Precision Oncology 3.
- Tong CWS, Wu M, Cho WCS (2018) Recent advances in the treatment of breast cancer. Front Oncol 8: 227.
- Bhullar KS, Lagarón NO, McGowan EM, Indu P, Amitabh J, et al. (2018) Kinase-targeted cancer therapies: Progress, challenges and future directions. Molecular Cancer, 48.
- Musumeci F, Greco C, Grossi G, Alessio M, Silvia S (2018) Recent studies on ponatinib in cancers other than chronic myeloid leukemia. Cancers (Basel) 10(11): 430.
- Bid HK, Roberts RD, Manchanda PK, Houghton J (2013) RAC1: An emerging therapeutic option for targeting cancer angiogenesis and metastasis. Mol Cancer Ther 12(10): 1925-1934.
- Hein AL, Post CM, Sheinin YM, Lakshmanan, Natarajan A, Enke CA, et al. (2016) RAC1 GTPase promotes the survival of breast cancer cells in response to hyper-fractionated radiation treatment. Oncogene 35(49): 6319-6329.
- Qiu J, Tao L, Wei Q, Zhang P (2018) Knockdown of Arf6 increases drug sensitivity and inhibits proliferation, migration and invasion in gastric cancer SGC-7901 cells. Oncol Lett 15(2): 2147-2152.
- Hui X, Mingping Q, Bingkun Z, Chenyang W, Niraj M, et al. (2017) Inhibition of RAB1A suppresses epithelial-mesenchymal transition and proliferation of triple-negative breast cancer cells. Oncol Rep 37(3): 1619-1626.
- Wang Z, Cheng Z, Chen S, Zhu XG, Gu YP, et al. (2018) Aberrant expression of Rab1A and its prognostic significance in human colorectal cancer. Eur Rev Med Pharmacol Sci 22(14): 4509-4517.
- Dang CV (2012) MYC on the path to cancer. Cell 149(1): 22-35.
- Li Y, Wang H, Zheng XF (2015) Rab1 GTPases as oncogenes. Aging (Albany NY) 7(11): 897-898.
© 2020. Yi Lu. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






