- Submissions

Full Text
Novel Approaches in Cancer Study
Current Clinical Applications and Challenges of Circulating Tumor Cells
Qi Zheng1, Xixi Cheng2, Haoxiang Xuan2 and Shuyang Wang1*
1Department of Pathology, School of Basic Medical Sciences, Fudan University, 131 Dong’an Road, Shanghai 200032, Shanghai, China
2School of Clinical Medicine, Fudan University, 130 Dong’an Road, Shanghai 200032, Shanghai, China
*Corresponding author: Shuyang Wang, Department of Pathology, School of Basic Medical Sciences, Fudan University, 131 Dong’an Road, Shanghai 200032, Shanghai, China, Tel: +86 21-5423 7528-2122, Fax no: +86 21 5423 7596, Email: shuyangwang@fudan.edu.cn
Submission: July 31, 2020Published: September 16, 2020

ISSN:2637-773XVolume5 Issue2
Abstract
Analysis of circulating tumor cells (CTC) has received enormous attentions for its potential to obtain diverse information of tumors dynamically. Nowadays fast-developing detection technologies facilitate its biological research of tumor dissemination and clinical implications. This review is going to discuss clinical applications of CTC including prognosis and prediction of metastatic progression, surveillance of therapeutic response and identification of therapeutic targets and resistance mechanisms and to further introduce present detection technologies of CTC.
Keywords: Liquid biopsy; Circulating tumor cells; CTC
Abbreviations: CTC: Circulating Tumor Cell; CRPC: Castration-Resistant Prostate Cancer; NSCLC: Non-Small Cell Lung Cancer; CI: Confidence Interval; CDX: CTC-Derived eXplant
Introduction
Currently, circulating tumor cell (CTC), a crucial intermediate of metastasis from primary tumor to distant organ sites [1], has arisen enormous attention for its obvious diagnostic, prognostic and predictive potential for personalized medicine. Through dissemination of tumor cells to distant organ sites in bloodstream, it has been demonstrated that CTC can gain key characteristics required for metastasis such as recruitment of immune cells, inflammatory chemokines and cytokines, while still facing significant barriers including physical stress, oxidative stress and anoikis, resulting in its short half-life [2]. It is noted that not only CTC numbers can be quantified, but also changes in DNA, RNA, and protein levels describe multiple heterogeneities compared with nonmalignant cells. And present studies have proposed that both single CTC and CTC clusters contribute to metastasis and CTC clusters with epithelial and mesenchymal phenotypes may be more metastatic than single cells [3].
We are going to further discuss the clinical applications and detection technologies concerning CTC which attracts tremendous interests in the medical frontier from three aspects including prognosis and prediction of metastatic relapse and mortality, surveillance of therapeutic response and identification of therapeutic targets and resistance mechanisms.
Clinical Applications of CTC
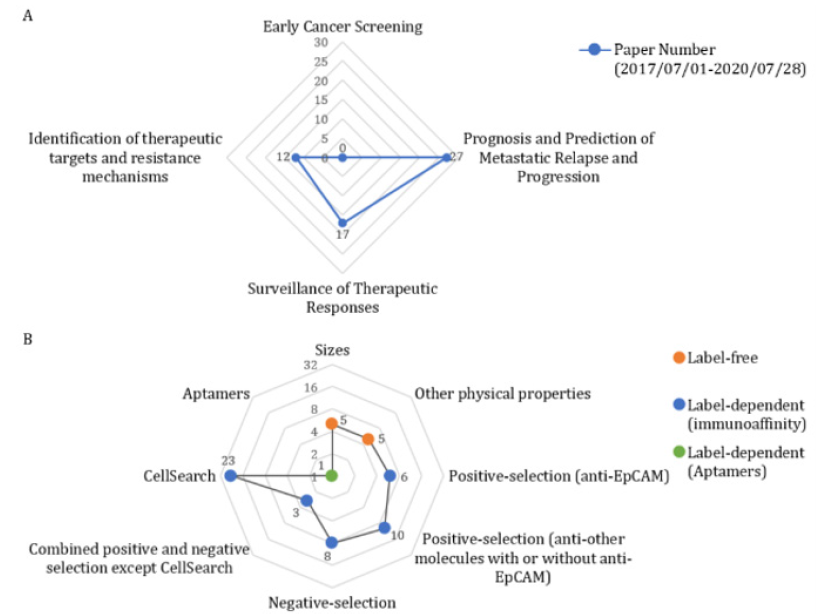
In this review, we collected published original research articles (impact factor, IF>10) in Pubmed in the recent three years and classified researches in each application subset (Figure 1) [2,4-57]. In consistent with its metastatic characteristics, we found that CTC might be more applicable in prognosis and surveillance of metastatic cancer rather than early cancer screening.
Figure 1: CTC-related publications counting via PubMed in the recent there years (IF>10). A. Comparison of published paper amount on early cancer screening (0), prognosis and prediction of metastatic progression(27) [4,32-57], surveillance of therapeutic responses(17) [15-31], and identification of therapeutic targets and resistance mechanisms(12) [2, 4-14], respectively. B. Comparison of published paper amount on different CTC-enrichment methodologies: size(5)[2,4,13,42,48], other physical properties(5)[10, 36, 64-66], positive selection(anti-EpCAM, 6)[19,22,34,43, 50,55], positive selection(anti-other molecules with or without anti-EpCAM, 10)[7,19,24,28,45,46,69,72], negative selection(8) [6,8,10,20,23,29,41,65], combined positive and negative selection expect CellSearh(3)[12,21,67], CellSearch(23) [5,9,11,15,17,25 27,32,35,37,38,44,47,49,51-54,56,77,78], and aptamers(1) [68].
Prognosis and prediction of metastatic relapses and progression
There are a number of studies presenting the compelling correlation between CTC and prognosis in patients with various tumor types, especially for assessment of survival in clinical trials. Netterberg’s team demonstrated that CTC counts could be useful for early predicting Overall Survival in patients with metastatic colorectal cancer [58]. Also in non-small cell lung cancer (NSCLC), one recent study said higher pretreatment CTC and persistence of CTC posttreatment were significantly associated with elevated risk of recurrence outside the targeted treatment site in patients with early-stage NSCLC treated with stereotactic body radiotherapy [39], while another retrospective assessment showed that cerebrospinal fluid CTC was correlated with risk of death (Hazard Ratio: 3.39, 95% confidence interval(CI): 1.01–11.37; P=0.048) in patients with leptomeningeal metastases from NSCLC [55], while . Not only CTC quantification are associated with prognosis, but also the compounds in CTC can be prognostic biomarkers. It was reported that out of 47 patients with aggressive variant prostate cancer from whom 257 individual CTC were sequenced (1-22 CTC/patient), twenty (42.6%) had concurrent 2+ tumor suppressor genes losses in at least one CTC in association with poor survival and increased genomic instability, inferred by high large-scale transitions scores [14].
Surveillance of therapeutic responses
At present, a large number of researches stated that CTC counts, and its molecular analysis could predict and monitor drug responses in metastatic cancer [4,27,29]. For example, in castration-resistant prostate cancer (CRPC), CTC increases were associated with worse prognosis, suggesting alternative therapies after three cycles of chemotherapy [59], and another Phase III clinical trial reported CTC number could serve as a response measure of Prolonged Survival for metastatic CRPC [16].
Identification of therapeutic targets and resistance mechanisms
In the past few years, characterizing of rare, heterogeneous CTC greatly provides thorough insight into metastasis and helps develop novel targeted therapies particularly by high throughput sequencing. Franses and their colleagues identified stemness gene LIN28B expression in CTC is prognostic for pancreatic ductal adenocarcinoma by RNA-seq and investigated LIN28B molecular mechanism on metastasis [60]. Recently, single cell sequencing enables to monitor mutation status for therapeutic resistance [4] and identify DNA methylation remodeling from CTC cluster to single CTC which was proved to enhance metastasis [61]. According to these excellent findings, molecular characterization of CTC provides a unique opportunity to determinate drivers of dissemination and guide prospective treatments targeting the “seeds” of metastasis. In addition, CTC-derived eXplant (CDX) and a CDX-derived cell line established by CTC are promising tools for exploring new strategies on metastasis or drug-resistance [61,62].
Detection Technologies of CTC
In practice, both single CTC and CTC clusters are extremely rare in blood, detection technology which directly determines its abundance and purity is the most challenging part for thorough biological studies and clinical applications as well of CTC. In order to increase the concentration of CTC by several log units, a great many enrichment methods have developed rapidly which can be divided into two main groups: label-free methods based on invasive capacity or physical properties such as size, deformity and density, or labeldependent ones including positive or negative selection depending on the immunoaffinity [63]. Particularly, CellSearch approved by FDA have been widely applied in dozens of clinical trials (Figure 1) [2,4,6-8,10,12,13,19-24,28,29,34,36,41-43,45,46,48,50,55,64- 72]. Nowadays, current strategies are prone to develop devices combining antibody cocktails with advanced techniques like immunomagnetic beads, nanoparticles or microfluidics to isolate and identify CTC for higher specificity and sensitivity [3,73,74].
Discussion and Outlook
CTC displaying similar molecular properties of primary tumor tissues and additional metastatic-associated changes can reveal the biology of the tumor dissemination that has not been clearly elucidated yet and guide the diagnosis, prognosis, monitoring and treatment of metastatic diseases. Due to its noninvasive and repeatable features, it has been validated as diagnostic and prognostic surrogates of tissue sampling and predictive indicators of recurrence and resistance for efficient therapeutic interventions [4,75]. Cancer screening at an asymptomatic stage always starts with determining the regulation in retrospective case-control trails and then setting cohort trails containing large populations requires long follow-up time to validate it. Practically, only few studies evaluated CTC as a biomarker for early cancer detection because it takes time for tumor cells to progress from primary site into blood and CTC might be zero or extremely low at an early stage. However, one group reported that CTC allowed early diagnosis of lung cancer in patients with chronic obstructive pulmonary disease [75], and another preliminary study has estimated that their CTC detection method based on RNA signature could enable a noninvasive early diagnosis of hepatocellular carcinoma in populations where viral hepatitis and cirrhosis are prevalent [76]. Though focusing on a population with high risk of developing cancer could speed up the long validation process, these studies need large further cohort to provide more evidences.
The biggest obstacle of CTC is its detection technologies. Basically, the heterogeneity in phenotypes and genotypes has made it challenging to directly capture CTC and raised the question whether the panel that we use to identify CTC is sensitive and specific enough for a certain tumor type. Label-free and aptamersdependent methods are novel isolations means that can overcome this shortage to some extent, but the potential applications of some highly sensitive detection methods need clinical trials with larger population to validate. More importantly, it is essential to evaluate and establish criteria for the clinical utility of CTC to assure its efficacy [77,78].
In spite of these challenges, these exciting findings on CTC highlight its crucial role during metastatic process as well as its significant application value of predicting prognosis or drugresponses and developing novel therapeutic strategies. We believe that CTC with these outstanding advantages promise to have a bright future.
Acknowledgement
The authors acknowledge grants from Shanghai Municipal Population and Family Planning Commission (201540251, SW) and National Natural Science Foundation of China (81972803, SW).
Conflicts of Interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
- Massague J, Obenauf AC (2016) Metastatic colonization by circulating tumour cells. Nature 529(7586): 298-306.
- Wei C, Yang C, Wang S, Shi D, Zhang C, et al. (2019) Crosstalk between cancer cells and tumor associated macrophages is required for mesenchymal circulating tumor cell-mediated colorectal cancer metastasis. Mol Cancer 18(1): 64.
- Chen K, Dopico P, Varillas J, Zhang J, George TJ, et al. (2019) Integration of lateral filter arrays with immunoaffinity for circulating-tumor-cell isolation. Angew Chem Int Ed Engl 58(23): 7606-7610.
- Pailler E, Faugeroux V, Oulhen M, Mezquita L, Laporte M, et al. (2019) Acquired resistance mutations to ALK inhibitors identified by single circulating tumor cell sequencing in ALK-rearranged non-small-cell lung cancer. Clin Cancer Res 25(22): 6671-6682.
- Sun YF, Guo W, Xu Y, Shi YH, Gong ZJ, et al. (2018) Circulating tumor cells from different vascular sites exhibit spatial heterogeneity in epithelial and mesenchymal composition and distinct clinical significance in hepatocellular carcinoma. Clin Cancer Res 24(3): 547-559.
- Grillet F, Bayet E, Villeronce O, Zappia L, Lagerqvist EL, et al. (2017) Circulating tumour cells from patients with colorectal cancer have cancer stem cell hallmarks in ex vivo Gut 66(10): 1802-1810.
- Lallo A, Frese KK, Morrow CJ, Sloane R, Gulati S, et al. (2018) The Combination of the PARP inhibitor olaparib and the WEE1 inhibitor AZD1775 as a new therapeutic option for small cell lung cancer. Clin Cancer Res 24(20): 5153-5164.
- Ebright RY, Lee S, Wittner BS, Niederhoffer KL, Nicholson BT, et al. (2020) Deregulation of ribosomal protein expression and translation promotes breast cancer metastasis. Science 367(6485): 1468-1473.
- Magbanua MJM, Rugo HS, Wolf DM, Hauranieh L, Roy R, et al. (2018) Expanded genomic profiling of circulating tumor cells in metastatic breast cancer patients to assess biomarker status and biology over time (CALGB 40502 and CALGB 40503, Alliance). Clin Cancer Res 24(6): 1486-1499.
- Drapkin BJ, George J, Christensen CL, Mino-Kenudson M, Dries R, et al. (2018) Genomic and functional fidelity of small cell lung cancer patient-derived xenografts. Cancer Discov 8(5): 600-615.
- Liu X, Taftaf R, Kawaguchi M, Chang YF, Chen W, et al. (2019) Homophilic CD44 interactions mediate tumor cell aggregation and polyclonal metastasis in patient-derived breast cancer models. Cancer Discov 9(1): 96-113.
- Boral D, Vishnoi M, Liu HN, Yin W, Sprouse ML, et al. (2017) Molecular characterization of breast cancer CTCs associated with brain metastasis. Nat Commun 8(1): 196.
- Adams DL, Adams DK, He J, Kalhor N, Zhang M, et al. (2017) Sequential tracking of PD-L1 expression and RAD50 induction in circulating tumor and stromal cells of lung cancer patients undergoing radiotherapy. Clin Cancer Res 23(19): 5948-5958.
- Malihi PD, Graf RP, Rodriguez A, Ramesh N, Lee J, et al. (2020) Single-cell circulating tumor cell analysis reveals genomic instability as a distinctive feature of aggressive prostate cancer. Clin Cancer Res 26(15): 4143-4153.
- Goodman CR, Seagle BL, Friedl TWP, Rack B, Lato K, et al. (2018) Association of circulating tumor cell status with benefit of radiotherapy and survival in early-stage breast cancer. JAMA Oncol 4(8): e180163.
- Heller G, McCormack R, Kheoh T, Molina A, Smith MR, et al. (2018) Circulating tumor cell number as a response measure of prolonged survival for metastatic castration-resistant prostate cancer: A comparison with prostate-specific antigen across five randomized phase III clinical trials. J Clin Oncol 36(6): 572-580.
- Magbanua MJM, Savenkov O, Asmus EJ, Ballman KV, Scott JH, et al. (2020) Clinical significance of circulating tumor cells in hormone receptor-positive metastatic breast cancer patients who received letrozole with or without bevacizumab. Clin Cancer Res.
- Graf RP, Hullings M, Barnett ES, Carbone E, Dittamore R, et al. (2020) Clinical utility of the nuclear-localized AR-V7 biomarker in circulating tumor cells in improving physician treatment choice in castration-resistant prostate cancer. Eur Urol 77(2): 170-177.
- Dong J, Jan YJ, Cheng J, Zhang RY, Meng M, et al. (2019) Covalent chemistry on nanostructured substrates enables noninvasive quantification of gene rearrangements in circulating tumor cells. Sci Adv 5(7): eaav9186.
- Kwan TT, Bardia A, Spring LM, Giobbie-Hurder A, Kalinich M, et al. (2018) A digital RNA signature of circulating tumor cells predicting early therapeutic response in localized and metastatic breast cancer. Cancer Discov 8(10): 1286-1299.
- Wei S, Guo C, He J, Tan Q, Mei J, et al. (2019) Effect of vein-first vs artery-first surgical technique on circulating tumor cells and survival in patients with non-small cell lung cancer: A randomized clinical trial and registry-based propensity score matching analysis. JAMA Surg 154(7): e190972.
- Mastoraki S, Strati A, Tzanikou E, Chimonidou M, Politaki E, et al. (2018) ESR1 methylation: A liquid biopsy-based epigenetic assay for the follow-up of patients with metastatic breast cancer receiving endocrine treatment. Clin Cancer Res 24(6): 1500-1510.
- Li Y, Zhang X, Liu D, Gong J, Wang DD, et al. (2018) Evolutionary expression of HER2 conferred by chromosome aneuploidy on circulating gastric cancer cells contributes to developing targeted and chemotherapeutic resistance. Clin Cancer Res 24(21): 5261-5271.
- Tagawa ST, Antonarakis ES, Gjyrezi A, Galletti G, Kim S, et al. (2019) Expression of AR-V7 and ARv(567es) in circulating tumor cells correlates with outcomes to taxane therapy in men with metastatic prostate cancer treated in TAXYNERGY. Clin Cancer Res 25(6): 1880-1888.
- Slovin S, Hussain S, Saad F, Garcia J, Picus P, et al. (2019) Pharmacodynamic and clinical results from a phase I/ii study of the hsp90 inhibitor onalespib in combination with abiraterone acetate in prostate cancer. Clin Cancer Res 25(15): 4624-4633.
- Trapp E, Janni W, Schindlbeck C, Jückstock J, Andergassen U, et al. (2019) Presence of circulating tumor cells in high-risk early breast cancer during follow-up and prognosis. J Natl Cancer Inst 111(4): 380-387.
- Armstrong AJ, Halabi S, Luo J, Nanus DM, Giannakakou P, et al. (2019) Prospective multicenter validation of androgen receptor splice variant 7 and hormone therapy resistance in high-risk castration-resistant prostate cancer: The PROPHECY Study. J Clin Oncol 37(13): 1120-1129.
- Antonarakis ES, Tagawa ST, Galletti G, Worroll D, Ballman K, et al. (2017) Randomized, noncomparative, phase II trial of early switch from docetaxel to cabazitaxel or vice versa, with integrated biomarker analysis, in men with chemotherapy-naïve, metastatic, castration-resistant prostate cancer. J Clin Oncol 35(28): 3181-3188.
- Miyamoto DT, Lee RJ, Kalinich M, Li Causi JA, Zheng Y, et al. (2018) An RNA-based digital circulating tumor cell signature is predictive of drug response and early dissemination in prostate cancer. Cancer Discov 8(3): 288-303.
- Autio KA, Dreicer R, Anderson J, Garcia JA, Alva A, et al. (2018) Safety and efficacy of BIND-014, a docetaxel nanoparticle targeting prostate-specific membrane antigen for patients with metastatic castration-resistant prostate cancer: A phase 2 clinical trial. JAMA Oncol 4(10): 1344-1351.
- Ignatiadis M, Litière S, Rothe F, Riethdorf S, Proudhon C, et al. (2018) Trastuzumab versus observation for HER2 nonamplified early breast cancer with circulating tumor cells (EORTC 90091-10093, BIG 1-12, Treat CTC): A randomized phase II trial. Ann Oncol 29(8): 1777-1783.
- De Laere B, Rajan P, Grönberg H, Dirix L, Lindberg J (2019) Androgen receptor burden and poor response to abiraterone or enzalutamide in TP53 wild-type metastatic castration-resistant prostate cancer. JAMA Oncol 5(7): 1060-1062.
- Sparano J, O'Neill A, Alpaugh K, Wolff AC, Northfelt DW, et al. (2018) Association of circulating tumor cells with late recurrence of estrogen receptor-positive breast cancer: A secondary analysis of a randomized clinical trial. JAMA Oncol 4(12): 1700-1706.
- Radovich M, Jiang G, Hancock BA, Chitambar C, Nanda R, et al. (2020) Association of circulating tumor dna and circulating tumor cells after neoadjuvant chemotherapy with disease recurrence in patients with triple-negative breast cancer: Preplanned secondary analysis of the BRE12-158 randomized clinical trial. JAMA Oncol: e202295.
- Rossi G, Mu Z, Rademaker AW, Austin LK, Strickland KS, et al. (2018) Cell-free DNA and circulating tumor cells: comprehensive liquid biopsy analysis in advanced breast cancer. Clin Cancer Res 24(3): 560-568.
- Rugo HS, Cortes J, Awada A, O'Shaughnessy J, Twelves C, et al. (2018) Change in topoisomerase 1-positive circulating tumor cells affects overall survival in patients with advanced breast cancer after treatment with etirinotecan pegol. Clin Cancer Res 24(14): 3348-3357.
- Paoletti C, Miao J, Dolce EM, Darga EP, Repollet MI, et al. (2019) Circulating tumor cell clusters in patients with metastatic breast cancer: A SWOG S0500 translational medicine study. Clin Cancer Res 25(20): 6089-6097.
- Hugenschmidt H, Labori KJ, Brunborg C, Verbeke CS, Seeberg LT, et al. (2020) Circulating tumor cells are an independent predictor of shorter survival in patients undergoing resection for pancreatic and periampullary adenocarcinoma. Ann Surg 271(3): 549-558.
- Frick MA, Feigenberg SJ, Jean-Baptiste SR, Aguarin LA, Mendes A, et al. (2020) Circulating tumor cells are associated with recurrent disease in patients with early-stage non-small cell lung cancer treated with stereotactic body radiotherapy. Clin Cancer Res 26(10): 2372-2380.
- Bidard FC, Michiels S, Riethdorf S, Mueller V, Esserman LJ, et al. (2018) Circulating tumor cells in breast cancer patients treated by neoadjuvant chemotherapy: A meta-analysis. J Natl Cancer Inst 110(6): 560-567.
- Guo W, Sun YF, Shen MN, Ma XL, Wu J, et al. (2018) Circulating tumor cells with stem-like phenotypes for diagnosis, prognosis, and therapeutic response evaluation in hepatocellular carcinoma. Clin Cancer Res 24(9): 2203-2213.
- Lorente D, Olmos D, Mateo J, Dolling D, Bianchini D, et al. (2018) Circulating tumour cell increase as a biomarker of disease progression in metastatic castration-resistant prostate cancer patients with low baseline CTC counts. Ann Oncol 29(7): 1554-1560.
- Antonarakis ES, Lu C, Luber B, Wang H, Chen Y, et al. (2017) Clinical significance of androgen receptor splice variant-7 mRNA detection in circulating tumor cells of men with metastatic castration-resistant prostate cancer treated with first- and second-line abiraterone and enzalutamide. J Clin Oncol 35(19): 2149-2156.
- Sharp A, Welti JC, Lambros MBK, Dolling D, Rodrigues DN, et al. (2019) Clinical utility of circulating tumour cell androgen receptor splice variant-7 status in metastatic castration-resistant prostate cancer. Eur Urol 76(5): 676-685.
- Ma B, King AD, Leung L, Wang K, Poon A, et al. (2017) Identifying an early indicator of drug efficacy in patients with metastatic colorectal cancer-a prospective evaluation of circulating tumor cells, 18F-fluorodeoxyglucose positron-emission tomography and the RECIST criteria. Ann Oncol 28(7): 1576-1581.
- Effenberger KE, Schroeder C, Hanssen A, Wolter S, Eulenburg C, et al. (2018) Improved risk stratification by circulating tumor cell counts in pancreatic cancer. Clin Cancer Res 24(12): 2844-2850.
- Su Z, Wang Z, Ni X, Duan J, Gao Y, et al. (2019) Inferring the evolution and progression of small-cell lung cancer by single-cell sequencing of circulating tumor cells. Clin Cancer Res 25(16): 5049-5060.
- Xu L, Mao X, Guo T, Chan PY, Shaw G, et al. (2017) The novel association of circulating tumor cells and circulating megakaryocytes with prostate cancer prognosis. Clin Cancer Res 23(17): 5112-5122.
- Riethdorf S, Müller V, Loibl S, Nekljudova V, Weber K, et al. (2017) Prognostic impact of circulating tumor cells for breast cancer patients treated in the neoadjuvant "geparquattro" trial. Clin Cancer Res 23(18): 5384-5393.
- Strati A, Koutsodontis G, Papaxoinis G, Angelidis I, Zavridou M, et al. (2017) Prognostic significance of PD-L1 expression on circulating tumor cells in patients with head and neck squamous cell carcinoma. Ann Oncol 28(8): 1923-1933.
- Tay RY, Fernández-Gutiérrez F, Foy V, Burns K, Pierce J, et al. (2019) Prognostic value of circulating tumour cells in limited-stage small-cell lung cancer: Analysis of the concurrent once-daily versus twice-daily radiotherapy (CONVERT) randomised controlled trial. Ann Oncol 30(7): 1114-1120.
- Konczalla L, Ghadban T, Effenberger KE, Wöstemeier A, Riethdorf S, et al. (2019) Prospective comparison of the prognostic relevance of circulating tumor cells in blood and disseminated tumor cells in bone marrow of a single patient's cohort with esophageal cancer. Ann Surg.
- Lindsay CR, Faugeroux V, Michiels S, Pailler E, Facchinetti F, et al. (2017) A prospective examination of circulating tumor cell profiles in non-small-cell lung cancer molecular subgroups. Ann Oncol 28(7): 1523-1531.
- Chemi F, Rothwell DG, McGranahan N, Gulati S, Abbosh C, et al. (2019) Pulmonary venous circulating tumor cell dissemination before tumor resection and disease relapse. Nat Med 25(10): 1534-1539.
- Nevel KS, DiStefano N, Lin X, Skakodub A, Ogilvie SQ, et al. (2020) A retrospective, quantitative assessment of disease burden in patients with leptomeningeal metastases from non-small-cell lung cancer. Neuro Oncol 22(5): 675-683.
- Magbanua MJM, Yau C, Wolf DM, Lee JS, Chattopadhyay A, et al. (2019) Synchronous detection of circulating tumor cells in blood and disseminated tumor cells in bone marrow predicts adverse outcome in early breast cancer. Clin Cancer Res 25(17): 5388-5397.
- De Laere B, Oeyen S, Mayrhofer M, Whitington T, van Dam PJ, et al. (2019) TP53 outperforms other androgen receptor biomarkers to predict abiraterone or enzalutamide outcome in metastatic castration-resistant prostate cancer. Clin Cancer Res 25(6): 1766-1773.
- Netterberg I, Karlsson MO, Terstappen LW, Koopman M, Punt CJA, et al. (2020) Comparing circulating tumor cell counts with dynamic tumor size changes as predictor of overall survival - A quantitative modeling framework. Clin Cancer Res.
- Vogelzang NJ, Fizazi K, Burke JM, De Wit R, Bellmunt J, et al. (2017) Circulating tumor cells in a phase 3 study of docetaxel and prednisone with or without lenalidomide in metastatic castration-resistant prostate cancer. Eur Urol 71(2): 168-171.
- Franses JW, Philipp J, Missios P, Bhan I, Liu A, et al. (2020) Pancreatic circulating tumor cell profiling identifies LIN28B as a metastasis driver and drug target. Nat Commun 11(1): 3303.
- Gkountela S, Castro-Giner F, Szczerba BM, Vetter M, Landin J, et al. (2019) Circulating tumor cell clustering shapes DNA methylation to enable metastasis seeding. Cell 176(1-2): 98-112.
- Faugeroux V, Pailler E, Oulhen M, Deas O, Brulle-Soumare L, et al. (2020) Genetic characterization of a unique neuroendocrine trans differentiation prostate circulating tumor cell-derived eXplant model. Nat Commun 11(1): 1884.
- Song Y, Shi Y, Huang M, Wang W, Wang Y, et al. (2019) Bioinspired engineering of a multivalent aptamer-functionalized nanointerface to enhance the capture and release of circulating tumor cells. Angew Chem Int Ed Engl 58(8): 2236-2240.
- Sun M, Xu J, Shamul JG, Lu X, Husain S, et al. (2019) Creating a capture zone in microfluidic flow greatly enhances the throughput and efficiency of cancer detection. Biomaterials 197: 161-170.
- Heitzer E, Speicher MR (2018) Digital circulating tumor cell analyses for prostate cancer precision oncology. Cancer Discov 8(3): 269-271.
- Chen Y, Peng J, Lai Y, Wu B, Sun L, et al. (2019) Ultrasensitive label-free detection of circulating tumor cells using conductivity matching of two-dimensional semiconductor with cancer cell. Biosens Bioelectron 142: 111520.
- Liu X, Li J, Cadilha BL, Markota A, Voigt C, et al. (2019) Epithelial-type systemic breast carcinoma cells with a restricted mesenchymal transition are a major source of metastasis. Sci Adv 5(6): eaav4275.
- Abate MF, Jia S, Ahmed MG, Li X, Lin L, et al. (2019) Visual quantitative detection of circulating tumor cells with single-cell sensitivity using a portable microfluidic device. Small 15(14): e1804890.
- Bernemann C, Humberg V, Thielen B, Steinestel J, Chen X, et al. (2019) Comparative analysis of AR variant AR-V567es mRNA detection systems reveal eminent variability and questions the role as a clinical biomarker in prostate cancer. Clin Cancer Res 25(13): 3856-3864.
- Myung JH, Eblan MJ, Caster JM, Park SJ, Poellmann MJ, et al. (2018) Multivalent binding and biomimetic cell rolling improves the sensitivity and specificity of circulating tumor cell capture. Clin Cancer Res 24(11): 2539-2547.
- Bu J, Nair A, Kubiatowicz LJ, Poellmann MJ, Jeong WJ, et al. (2020) Surface engineering for efficient capture of circulating tumor cells in renal cell carcinoma: From nanoscale analysis to clinical application. Biosens Bioelectron 162: 112250.
- Agerbæk M, Bang-Christensen, Yang MH, Clausen TM, Pereira MA, et al. (2018) The VAR2CSA malaria protein efficiently retrieves circulating tumor cells in an EpCAM-independent manner. Nat Commun 9(1): 3279.
- Wu LL, Zhang ZL, Tang M, Zhu DL, Dong XJ, et al. (2020) Spectrally combined encoding for profiling heterogeneous circulating tumor cells using a multifunctional nanosphere-mediated microfluidic platform. Angew Chem Int Ed Engl 59(28): 11240-11244.
- Bankó P, Lee SY, Nagygyörgy V, Zrínyi M, Chae CH, et al. (2019) Technologies for circulating tumor cell separation from whole blood. J Hematol Oncol 12(1): 48.
- Ilie M, Hofman V, Long-Mira E, Selva E, Vignaud JM, et al. (2014) Sentinel circulating tumor cells allow early diagnosis of lung cancer in patients with chronic obstructive pulmonary disease. PLoS One 9(10): e111597.
- Kalinich M, Bhan I, Kwan TT, Miyamoto DT, Javaid S, et al. (2017) An RNA-based signature enables high specificity detection of circulating tumor cells in hepatocellular carcinoma. Proc Natl Acad Sci USA 114(5): 1123-1128.
- Tamminga M, de Wit S, vande Wauwer C, vanden Bos H, Swennenhuis JF, et al. (2020) Analysis of released circulating tumor cells during surgery for non-small cell lung cancer. Clin Cancer Res 26(7): 1656-1666.
- Lambros MB, Seed G, Sumanasuriya S, Gil V, Crespo M, et al. (2018) Single-cell analyses of prostate cancer liquid biopsies acquired by apheresis. Clin Cancer Res 24(22): 5635-5644.
© 2020. Shuyang Wang. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






