- Submissions

Full Text
Novel Approaches in Cancer Study
Expression and Gene Regulation Network of TFF1 in Esophageal Carcinoma
Wen Li1, Jingyu Li2, Dezhi Song1, Xingxin Gao3, Yingwen Huang4 and Xiaolong Li1*
1School of Preclinical Medicine, China
2Department of Otolaryngology Head and Neck Surgery, China
3Department of Burn and Plastic Surgery, China
4Department of Central Laboratory, China
*Corresponding author: Xiaolong Li, Department of Cell Biology and Genetics, School of Preclinical Medicine, Guangxi 530021, P.R. China and Yingwen Huang, Department of Central Laboratory, 89-9 Dongge Road, Nanning, Guangxi 530000, P.R. China
Submission: March 11, 2020Published: July 20, 2020

ISSN:2637-773XVolume5 Issue1
Abstract
TFF1, one member of the trefoil factor family (TFFs), is an antiproteinolytic peptide. Abnormal TFF1 expression is associated with carcinogenesis. In order to investigate the expression of TFF1 in esophageal carcinoma and its potential gene regulatory network. We used sequencing data from the Cancer Genome Atlas database and Gene Expression Omnibus, analyzed TFF1 expression and gene regulation networks in esophageal carcinoma (ESCA). TFF1 expression profiling was analyzed using Oncomine TM, while TFF1 mutation and related functional networks were identified using cBioPortal. Linked Omics was used to identify differential gene expression with TFF1 and to analyze Gene Ontology and Kyoto Encyclopedia of Genes and Genomes pathways. We found that TFF1 is overexpressed in ESCA, and deletion is the most common TFF1 mutation type in ESCA, and TFF1 gene mutation may also significantly affect the prognosis of ESCA patients. Functional network analysis showed that TFF1 may play a role in ESCA by participating in NF-κB signaling pathway and Hippo signaling pathway. Our results demonstrate that data mining efficiently reveals information about TFF1 expression and potential regulatory networks in ESCA, laying a foundation for further study of the role of TFF1 in carcinogenesis.
Keywords: Trefoil factor family; Esophageal carcinoma; Bioinformatics
Introduction
Esophageal carcinoma (ESCA) has been ranked the seventh position in the world cancer incidence rate, and the mortality rate is sixth [1]. According to histological classification, ESCA includes squamous cell carcinoma (ESCC), adenocarcinoma (EAC) and undifferentiated carcinoma. Among them, squamous cell carcinoma is the most popular type of esophageal carcinoma [2]. In Asia, more than 90% of esophageal carcinoma is ESCC, while EAC mostly occurs in European and American countries [3]. China is one of the countries with a high incidence of esophageal cancer, most of which are ESCC. It has been reported that ESCC ranks fifth in cancer incidence and fourth in mortality, in China mainland [4]. ESCA, as the main histopathological manifestation of malignant tumors, is a fatal malignant tumor with low survival rate [5]. Despite advanced diagnosis and treatment methods, most cases are diagnosed as advanced and have a poor prognosis due to rapid metastasis [6]. To date, several studies have investigated that more than 50% of patients start metastasis at an early diagnosis [3]. According to the stage of metastasis, malignant tumors can be divided into local metastases, local metastases, and distant metastases. In addition, it has been determined that the distant metastatic stage can cause death in approximately 50% of patients with malignancies [7]. In addition, the prognosis of patients with ESCA is miserable due to late diagnosis and poor response to treatment, and the limit of 5-year survival rate is 15% [4,8-10]. Consequently, it is a pressing and essential work to find biomarkers for preclinical diagnosis and hazard ranking of ESCA. It is possible to discover new ESCA-related biomarkers by screening gene networks related to tumor formation and progression.
Members of the trefoil factor family (TFF) include TFF1 or gastric peptide (pS2), TFF2 or spasmolytic peptide (SP), and TFF3 or intestinal trefoil factor. Structurally, a cysteine-rich motif is a conserved structure for members of the TFF family. This motif can form a tricyclic structure through a disulfide bond, the so-called “three-leaf” domain. Previous studies have found that gastrointestinal mucosal epithelial cells express TFFs at high levels, and TFFs are conducive to improving the ability of mucosal cells to resist damage and protecting the structural integrity of mucosal epithelium [11]. However, the pathophysiological state of the tissue will also greatly affect the physiological activity of TFFs [12]. Due to the various expression levels of TFFs in tumors and different stages of tumor development, its role in tumorigenesis is still controversial. TFF1 was originally found in breast cancer cells [13]. Some studies have suggested that TFF1 is a promoter of cancer development [14,15], other research has found that TFF1 is a predictor of positive response to hormone therapy in breast cancer, however [16,17]. In addition, a number of researches in different tumor tissues, including colon, pancreatic, and ovarian cancer, have indicated that the survival, invasion, and metastasis of tumor cells may be regulated by TFF1 [18-20]. Furthermore,
TFF1 knockout mice have a high incidence of pyloric adenoma, in this trend, 30% of them boosted malignant gastric cancer [21].
These previous results suggest that TFF1 may be a new tumor marker. However, the expression of TFF1 in esophageal cancer and its regulated gene network have not been reported. Therefore, the expression characteristics and mutation rate of TFF1 in ESCA were analyzed in this study, based on data from multiple public databases including The Cancer Genome Atlas (TCGA). Furthermore, TFF1-related genomic changes and functional regulatory networks in ESCA have been described through a multidimensional analysis method. Consequently, our findings may provide novel biomarkers and strategies fronting early diagnosing and intervention of ESCA.
Materials and Methods
Oncomine analysis
TFF1 mRNA expression in ESCA was analyzed in Oncome 4.5 database. Oncomine (www.oncomine.org) is currently the most thorough database and comprehensive data mining platform for tumor-related gene research, including 715 gene expression data sets and data from 86733 cancer and normal tissues [22]. In this study, differences in TFF1 transcription levels among specific tumors and adjoining normal tissues were acquired from the Oncomine database. A series of ESCA studies including Kim's, kimchi's and Hao's were used in this study. Expression profiling of TFF1 in ESCA was compared with that in normal tissue. The t test was applied for analyzing the difference between transcription levels, and a p value less than 0.01 was regarded as statistically significant. The mRNA data was resolved using the following thresholds: 1.5 as basic fold difference and 10% gene alignment ratio.
GEPIA analysis
The expression of TFF1 in esophageal cancer tissues and normal tissues was assessed using the Gene Expression Profiling Interactive Analysis (GEPIA) platform. GEPIA is a web-based tool that enables the interactive analysis of cancer and normal gene expression using data from The Tumor Genome Map (TCGA) and Genotype Tissue Expression (GTEx) [23]. On this basis, the analysis of box diagram and tumor stage diagram was carried out. If the p value was less than 0.01, the difference between results was considered as statistically significant.
c-BioPortal analysis
The c-BioPortal is an open-access database that integrates and simplifies the contents of multiple cancer genome databases including TCGA, International Cancer Genome Consortium (ICGC) and GEO, and provides a friendly and visual interface. Here in this study c-BioPortal was used for resolving the characteristic of TFF1 in ESCA samples from TCGA. The search parameters included mutation and mRNA expression. The oncoprint tab displays an overview of the genetic mutations in each sample in TFF1. And the network visualizes the biological interaction network of TFF1 from the public pathway database, using color coding and screening options based on the frequency of each gene genome change. These include neighboring genes with high frequency of change. Kaplan Meier chart was used to show TFF1 gene mutation and its correlation with OS in ESCA patients. Log-rank test was performed to determine the significance of the difference among survival curves. The p value less than 0.05 regarded with a statistical difference between these results.
Analysis based on linked omics database
These 32 TCGA cancer-related cubes included in this study were dissected using the online database Linked Omics [24]. The link finder module of linked omics was applied to research the differentially expressed genes related to TFF1 in the TCGA ESCA cohort. The results were statistically analyzed by Pearson correlation coefficient. All the results are presented graphically in the volcanic map, thermal map or scatter map. The link interpreter module of linked omics analyzes the pathways and networks of differentially expressed genes. The data in link finder results are signed and sorted in Web Gestalt [25], and go (CC, BP, MF) and KEGG pathway are analyzed by GSEA.
Result
TFF1 mRNA expression in cancers
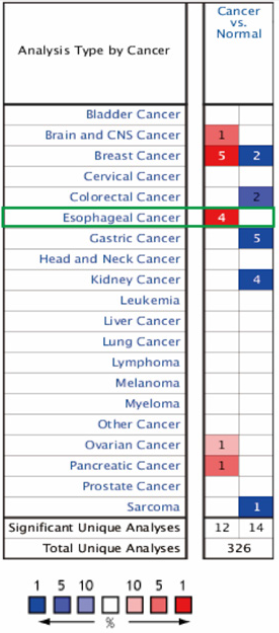
The main function of the Oncomine database is to analyze gene expression differences, correlation between gene expression and clinical, and co-expression of multiple genes. Data from the Oncomine database was used to assess the profiling of TFF1 mRNA expression in different tumor tissues as well as normal clinical specimens. In this study, 650 data sets, including 80, 551 samples were included. The results show that TFF1 mRNA expression was significantly elevated in head and central nervous system tumors, breast, esophageal, ovarian, and pancreatic cancers, while downregulated in colorectal, kidney and malignant mesenchymal tumors (Figure 1). Thus, there are a number of notable differences in TFF1 mRNA expression among diverse tumor types.
Figure 1: Pooled analyses on the mRNA expression of TFF1 in various carcinoma types. The mRNA expression of TFF1 (cancer vs. corresponding normal tissue) was evaluated using the Oncomine database (red represents significant overexpression and blue represents reduced expression). The following parameters were used as thresholds: P<1×104, fold-change >2 and gene ranking in the top 10%.
The Expression of TFF1 in ESCA
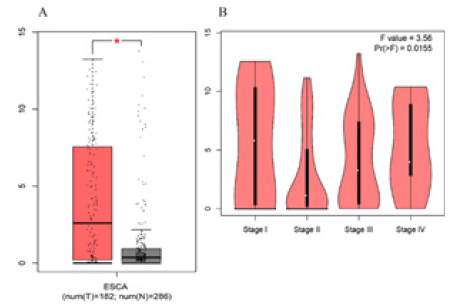
Two databases, Gene Expression Omnibus (GEO) and TCGA were used in order to evaluate the difference in the transcription level of TFF1 in different ESCA tissues. Data from the Oncomine 4.5 database show that the level of TFF1 mRNA expression in ESCA is raised significantly than that in normal tissues (p<0.05=. The fold changes were all greater than 2 and TFF1 was ranked in the top 1% based on mRNA expression (Figure 2). Based on the analysis of 182 esophageal cancer (ESCA) samples in the TCGA database, TFF1 also maintained high transcription standard in each tumor stage subgroup, and the highest level was found in stage 1 (Figure 3). Therefore, TFF1 expression may be a potential diagnostic indicator of ESCA.
Genomic mutation analysis of TFF1 in ESCA
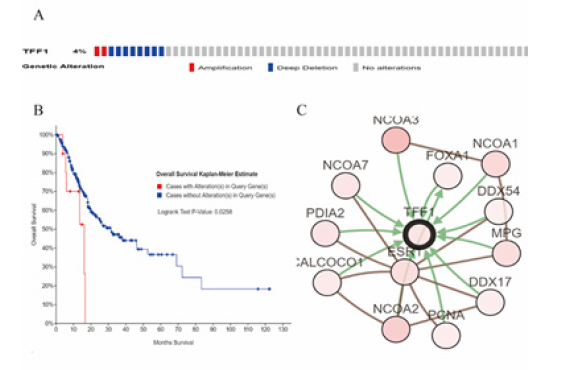
Based on the sequencing data of ESCA patients from the TCGA database, cBioPortal was applied in analyzing the type and frequency of TFF1 changes in ESCA. TFF1 was altered in 10 of the 265 ESCA patients (Figure 4A). These changes were deleted in 8 cases (3%) and amplified in 2 cases (1%). Therefore, deletion is the most common type of TFF1 mutation in ESCA. In addition, as shown in (Figure 4B), Kaplan-Meier plots indicate that TFF1 gene mutations are companied with shorter OS in ESCA patients (p = 0.0258). These results suggest that mutations in the TFF1 gene may also significantly affect the prognosis of ESCA patients.
Figure 2: TFF1 transcription in Esophagus carcinoma (Oncomine). Levels of TFF1 mRNA were significantly higher in Esophagus carcinoma than in normal tissue. Shown are fold change, associated p values, based on Oncomine 4.5 analysis. (A–C) Box plot showing TFF1 mRNA levels in, respectively, Kim Esophagus, Kimchi Esophagus, the Hao Esophagus.
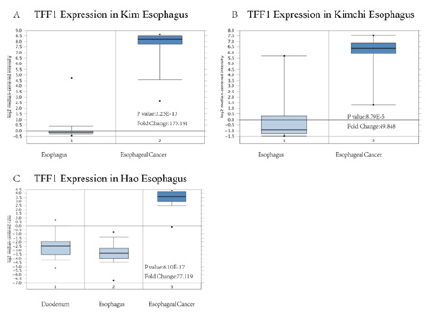
Figure 3: TFF1 transcription in subgroups of patients with Esophagus carcinoma, stratified based on Stage (GEPIA). (A) Boxplot showing relative expression of TFF1 in normal and ESCA samples. (B) Expression of TFF1 in ESCA based on individual cancer stages.
To assess the biological interaction network of TFF1 in ESCA, gene network toolkit in cBioPortal to further analyze the genes that interact with TFF1 in lung cancer. The analysis results show that the network contains 13 nodes, including 1 query gene TFF1 and 12 TFF1 neighboring genes. The type of interaction is derived from BioPAX: blue connections indicate that the first protein is involved in regulating the second protein, while red connections indicate that these proteins are parts of the same complex. The shade of the color represents the degree of mutation. The darker red nodes including nuclear receptor coactivators 1, 2, 3, estrogen receptor 1, and N-methylated purine DNA glycosylase, these genes have a higher degree of mutation than other genes in EACA (Figure 4C).
Figure 4: Visual summary of TFF1 alterations biological interaction network and TFF1 association with OS in Esophagus carcinoma (cBioPortal). (A) OncoPrint of TFF1 alterations in ESCA. The OncoPrint provides an overview of genomic alterations in TFF1 affecting individual samples (columns) in ESCA from the TCGA. The different types of genetic alterations are highlighted in different colors. (B) Genetic alterations in TFF1 were associated with shorter OS(P<0.05). (C) Network view of the TFF1 neighborhood in ESCA. TFF1 are seed genes (indicated with thick border), and all other genes are automatically identified as altered in ESCA.
Analysis of TFF1-related co-expressed genes in ESCA
Figure 5: Genes differentially expressed in correlation with TFF1 in Esophagus carcinoma (Linked Omics). (A) A Pearson test was used to analyze correlations between TFF1, and genes differentially expressed in ESCA. (B) Heat maps showing genes positively and negatively correlated with TFF1 in ESCA (TOP 50). (C) Red indicates positively correlated genes and green indicates negatively correlated.
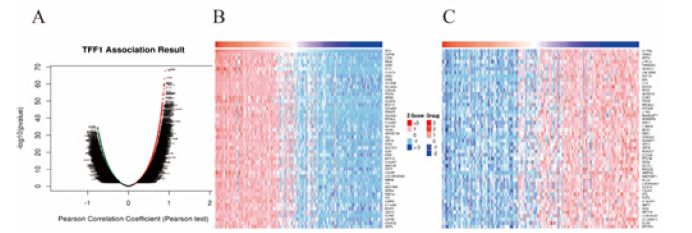
The mRNA data of 184 ESCA patients from TCGA were analyzed using the Linked Omics functional module. As present in Figure 5A and Figure 5B, 5370 genes (red dots) are significantly positively correlated with TFF1 while 896 genes (green dots) are negatively correlated with TFF1. Furthermore, 50 important genes that are negatively or positively correlated with TFF1 are shown in Figure 5C. These results indicate that TFF1 has a comprehensive influence on the transcriptome. Figure 6 shows that there are strong correlations between the high expression of TFF1 and expression of CAPN8 (Calpain 8, Pearson correlation coefficient = 0.90, p = 1.702 e-68), CTSE (Cathepsin E, Pearson correlation coefficient = 0.89, p = 6.33e-64), REG4 (Regenerative gene 4, Pearson correlation coefficient = 0.88, p = 7.97e-61), suggesting that TFF1 may participate in tumorigenesis and development through regulating the expression of CAPN8, CTSE and REG4.
Analysis of GO and KEGG pathways of TFF1-related co-expressed genes in ESCA
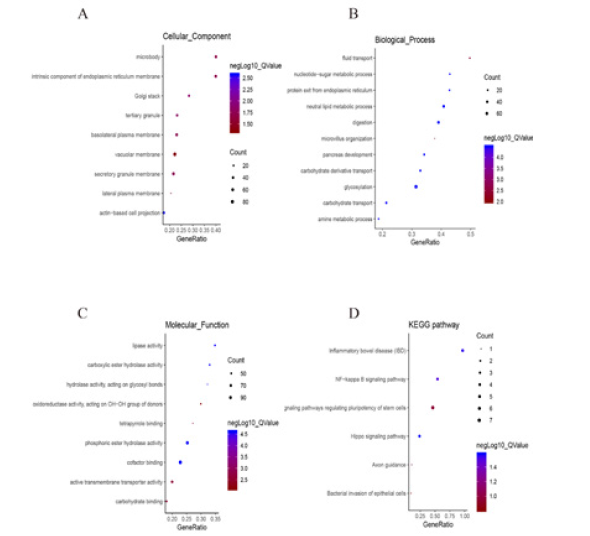
The GO analysis based on the Gene Set Enrichment Analysis (GSEA) indicated that the TFF1 related gene polymorphisms are mainly located in the microsome, endoplasmic reticulum membrane and Golgi apparatus, and involved in cell transport, nucleotide-sugar metabolism and endoplasm chiefly. Their molecular functions mainly include triggering lipase activity, carboxylate hydrolase activity, and hydrolase activity acting on glycosyl bonds (Figure 7A-7C). On the other hand, KEGG pathway analysis shows that TFF1 may play a role in ESCA by participating in the signaling transduction of NF-κB and Hippo pathway (Figure 7D).
Figure 6: Gene expression correlation analysis for TFF1, CAPN8, CTSE and REG4(Linked Omics). The scatter plot shows Pearson correlation of TFF1 expression with expression of CAPN8 (A), CTSE (B), and REG4 (C).
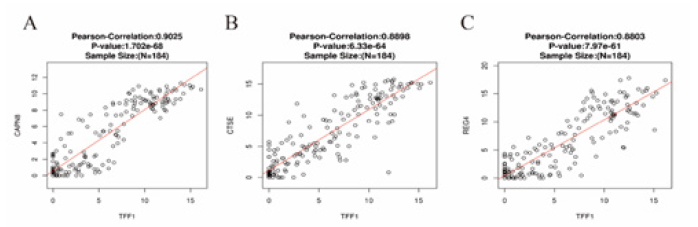
Figure 7: Significantly enriched GO annotations and KEGG pathways of TFF1 in Esophagus carcinoma. The significantly enriched GO annotations and KEGG pathways of TFF1 co-expression genes in ESCA were analyzed using GSEA. (A) Cellular components. (B) Biological processes. (C) Molecular functions. (D) KEGG pathway analysis.
Discussion
TFF1 is a protein expressed in various tissues such as the mucosal epithelium, it is one of the earliest members of the trefoil factor family (TFF) to be discovered, which contains a conserved tricyclic domain, called the TFF domain [26,27]. Maintaining the integrity of the mucosa in the gastrointestinal tract and promoting the regeneration of damaged mucosal epithelial cells are the primary functions of TFFs [11]. It has been reported that the expression of TFF1 in gastric cancer is impaired, and the incidence of gastric cancer in mice lacking TFF1 gene is enhanced [28]. In addition, TFF1 is expressed at a higher level in colon, pancreas and ovarian tumors than other tumor tissues, and is involved with stimulating cell survival, migration, invasiveness, and tumor spread. It has been shown that TFF1 is a potential tumor marker [19,20].
Our results here show that in ESCA patients, TFF1 is overexpressed, and its mRNA expression is significantly correlated with the individual cancer stage of patients. Moreover, we found that the mutation rate of TFF1 in ESCA patients was 4%, and the deletion was the most common type of TFF1 mutation in ESCA. The mutation of gene TFF1 may also significantly affect the prognosis of ESCA patients. Functional network analysis shows that TFF1 may play a role in ESCA by participating in the NF-κB signal pathway and Hippo signal pathway. The mechanism that led to the progress of ESCA has not been fully elucidated, and its early diagnosis biomarkers have not been confirmed and applied, which leads to poor diagnosis and treatment results. Our data shows that TFF1 expression in ESCA patients' tumor tissues is higher than in normal tissues, especially in esophageal cancer tumors at stage Ι. The results suggest that TFF1 is worthy of further research as a candidate biomarker for early diagnosis of ESCC. TFF1 is a mucosal protective factor, which is up regulated by stimuli of mucosal damage, promoting of mucosal repair and maintaining integrity [29]. TFF1 can suppress the activation of NF-κB signaling pathway by down-regulating the expression of inflammatory factors and anti-apoptotic proteins. Due to the lack of inhibition of TFF1, the NF-κB signaling pathway is over-activated in gastric cancer cells [30]. Similar to this, Functional network analysis shows that TFF1 may play a role in ESCA by participating in the NF-κB signal pathway and Hippo signal pathway. Upregulation of TFF1 expression in Barrett's esophagus (BE) has been reported to increase the incidence of esophageal adenocarcinoma, suggesting that upregulation of TFF1 is a characteristic of precancerous symptoms. Our results confirm previous observations [16,31]. Cause gastric acid reflux is closely related to BE. Mucosal damage due to gastric acid reflux may be one of the reasons for the upregulation of TFF1 expression in BE. In summary, these results note that TFF1 is enrolled in the occurrence and development of ESCA.
Recent studies have shown that EAC is similar to gastric adenocarcinoma in molecular changes, while ESCC is more analogous to head and neck squamous cell carcinomas [32]. But there are few studies on the TFF1 expression in cancers of head and neck. It has been found that squamous epithelial cells in the upper respiratory and digestive tracts of patients with head and neck cancer also undergo abnormal changes [33]. As a biomarker of early canceration, the expression of TFF1 in the esophageal mucosa may not only be used for the early diagnosis of squamous cell carcinoma of the head and neck, but also as a warning indicator for the second primary tumor of the esophagus (SPTE). In salivary gland tumors, the expression of TFF1, TFF2, and TFF3 is improved, while in oral squamous cell carcinoma the expression of TFF2, TFF3 is depressed, and expression of TFF1 is raised, compare to healthy tissue [34]. However, based on so few studies, we have not yet fully concluded the function of TFFs, especially in head and neck cancer, has led to ESCC, and this issue needs further exploration. In addition, functional network analysis indicates that TFF1 may play a role in ESCA by participating in the NF-κB signaling pathway, Hippo signaling pathway, however, the specific way through which it works need further confirmation.
In summary, our results show that high expression of TFF1 is found in ESCA patients, which is related to the stage of clinical cancer, with the highest expression in tumor stage Ⅰ. In addition, ESCA patients have also observed that TFF1 mutations are mainly deletions, and TFF1 gene mutations are associated with shorter OS in ESCA patients. These results indicate that TFF1 may be a new tumor marker and provide new targets and strategies for the diagnosis and treatment of ESCA.
Funding
- Natural Science Foundation of Guangxi Province (Grant number: 2017GXNSFAA198045).
- Natural Science Foundation of Guangxi Province (Grant number: 2017GXNSFAA198063).
Availability of Data and Materials
The datasets used and/or analyzed during the present study are available from the corresponding authors on reasonable request.
Author’s Contributions
WL and XL conceived, designed and analyzed the data. WL, XL, JL and YH prepared figures, wrote and revised the manuscript. DS and XG analyzed the data. All authors read and approved the final version of the manuscript.
Competing Interests
The authors declare that they have no competing interests.
References
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, et al. (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians 68: 394-424.
- Yoon JH, You BH, Park CH, Kim YJ, Nam JW, et al. (2018) The long noncoding RNA LUCAT1 promotes tumorigenesis by controlling ubiquitination and stability of DNA methyltransferase 1 in esophageal squamous cell carcinoma. Cancer Letters 417: 47-57.
- Yao J, Huang JX, Lin M, Wu ZD, Yu H, et al. (2016) Microarray expression profile analysis of aberrant long non-coding RNAs in esophageal squamous cell carcinoma. Int J Oncol 48: 2543-2557.
- Arnold M, Soerjomataram I, Ferlay J, Forman D (2015) Global incidence of oesophageal cancer by histological subtype in 2012. Gut 64: 381-387.
- He LR, Liu MZ, Li BK, Jia WH, Zhang Y, et al. (2010) High expression of EZH2 is associated with tumor aggressiveness and poor prognosis in patients with esophageal squamous cell carcinoma treated with definitive chemoradiotherapy. Int J Cancer 127: 138-147.
- Rui Q, Xu Z, Yang P, He Z (2015) Long noncoding RNA expression patterns in lymph node metastasis in colorectal cancer by microarray. Biomed Pharmacother 75: 12-18.
- Derrien T, Johnson R, Bussotti G, Tanzer A, Djebali S, et al. (2012) The GENCODE v7 catalog of human long noncoding RNAs: Analysis of their gene structure, evolution, and expression. Genome Res 22: 1775-1789.
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, et al. (2015) Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136: E359-E386.
- Allen JW, Richardson JD, Edwards MJ (1997) Squamous cell carcinoma of the esophagus: A review and update. Surg Oncol 6: 193-200.
- Knabe M, May A, Ell C (2016) Endoscopic resection for patients with mucosal adenocarcinoma of the esophagus. Minerva Gastroenterol Dietol 62: 281-295.
- Taupin D, Podolsky DK (2003) Trefoil factors: Initiators of mucosal healing. Nat Rev Mol Cell Biol 4: 721-732.
- Ghandi M, Huang FW, Valbuena JJ, Kryukov GV, Lo CC, et al. (2019) Next-generation characterization of the cancer cell line encyclopedia. Nature 569: 503-508.
- Masiakowski P, Breathnach R, Bloch J, Gannon F, Krust A, et al. (1982) Cloning of cDNA sequences of hormone-regulated genes from the MCF-7 human breast cancer cell line. Nucleic Acids Res 10: 7895-7903.
- Prest SJ, May FEB, Westley BR (2002) The estrogen-regulated protein, TFF1, stimulates migration of human breast cancer cells. FASEB J 16: 592-594.
- Amiry N, Kong X, Muniraj N, Kannan N, Grandison PM, et al. Trefoil factor-1 (TFF1) enhances oncogenicity of mammary carcinoma cells. Endocrinology 150: 4473-4483.
- Buache E, Etique N, Alpy F, Stoll I, Muckensturm M, et al. (2011) Deficiency in trefoil factor 1 (TFF1) increases tumorigenicity of human breast cancer cells and mammary tumor development in TFF1-knockout mice. Oncogene 30: 3261-3273.
- Henry JA, Nicholson S, Hennessy C, Lennard TW, May FE, et al. (1990) Expression of the oestrogen regulated pNR-2 mRNA in human breast cancer: Relation to oestrogen receptor mRNA levels and response to tamoxifen therapy. Br J Cancer 61: 32-38.
- Rodrigues S, Rodrigue CM, Attoub S, Fléjou JF, Bruyneel E, et al. (2006) Induction of the adenoma-carcinoma progression and Cdc25A-B phosphatases by the trefoil factor TFF1 in human colon epithelial cells. Oncogene 25: 6628-6636.
- Arumugam T, Brandt W, Ramachandran V, Moore TT, Wang H, et al. (2011) Trefoil factor 1 stimulates both pancreatic cancer and stellate cells and increases metastasis. Pancreas 40: 815-822.
- Zhao S, Ma Y, Huang X (2015) Trefoil factor 1 elevates the malignant phenotype of mucinous ovarian cancer cell through Wnt/β-catenin signaling. Int J Clin Exp Pathol 8: 10412-10419.
- Lefebvre O, Chenard MP, Masson R, Linares J, Dierich A, et al. (1996) Gastric mucosa abnormalities and tumorigenesis in mice lacking the pS2 trefoil protein. Science 274: 259-262.
- Rhodes DR, Sundaram KS, Mahavisno V, Varambally R, Jianjun Yu, et al. (2007) Oncomine 3.0: genes, pathways, and networks in a collection of 18,000 cancer gene expression profiles. Neoplasia 9(2): 166-180.
- Tang Z, Li C, Kang B, Gao G, Li C, et al. (2017) GEPIA: A web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res 45(W1): W98-W102.
- Vasaikar SV, Straub P, Wang J, Zhang B (2018) Linked omics: Analyzing multi-omics data within and across 32 cancer types. Nucleic Acids Res 46: D956-D963.
- Wang J, Vasaikar S, Shi Z, Greer M, Zhang B (2017) Web Gestalt 2017: A more comprehensive, powerful, flexible and interactive gene set enrichment analysis toolkit. Nucleic Acids Res 45(W1): W130-W137.
- Taupin D, Ooi K, Yeomans N, Giraud A (1996) Conserved expression of intestinal trefoil factor in the human colonic adenoma-carcinoma sequence. Lab Invest 75(1): 25-32.
- Hoffmann W, Jagla W (2002) Cell type specific expression of secretory TFF peptides: Colocalization with mucins and synthesis in the brain. Int Rev Cytol 213: 147-181.
- Yio X, Diamond M, Zhang JY, Weinstein H, Lu Hai W, et al. (2006) Trefoil factor family-1 mutations enhance gastric cancer cell invasion through distinct signaling pathways. Gastroenterology 130(6): 1696-1706.
- Taupin D, Pedersen J, Familari M, Cook G, Yeomans N, et al. (2001) Augmented intestinal trefoil factor (TFF3) and loss of pS2 (TFF1) expression precedes metaplastic differentiation of gastric epithelium. Lab Invest 81(3): 397-408.
- Thim L, May FEB (2005) Structure of mammalian trefoil factors and functional insights. Cell Mol Life Sci 62(24): 2956-2973.
- Fox CA, Sapinoso LM, Zhang H, Zhang W, McLeod HL, et al. (2005) Altered expression of TFF-1 and CES-2 in Barrett's Esophagus and associated adenocarcinomas. Neoplasia 7(4): 407-416.
- Nancarrow DJ, Clouston AD, Smithers BM, Gotley DC, Drew PA, et al. (2011) Whole genome expression array profiling highlights differences in mucosal defense genes in Barrett's esophagus and esophageal adenocarcinoma. PLoS One 6(7): e22513.
- Lim H, Kim DH, Jung HY, Gong EJ, Na HK, et al. (2015) Clinical significance of early detection of esophageal cancer in patients with head and neck cancer. Gut Liver 9(2): 159-165.
- Cancer Genome Atlas Research Network, Analysis Working Group: Asan U, Agency BCC, et al. (2017) Integrated genomic characterization of oesophageal carcinoma. Nature 541(7636): 169-175.
© 2020. Xiaolong Li. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






