- Submissions

Full Text
Novel Approaches in Cancer Study
Toll-Like Receptor Agonists in Cancer Therapy
Jonathan Zhang1, Chikezie O Madu2 and Yi Lu3*
1Departments of Biology and Advanced Placement Biology, White Station High School, Memphis, Tennessee 28117, USA, jonathanzhang53@gmail.com
2Departments of Biology and Advanced Placement Biology, White Station High School, Memphis, Tennessee 28117, USA, maduco@scsk12.org
3Department of Pathology and Laboratory Medicine, University of Tennessee Health Science Center, Memphis, TN 38163, USA, ylu@uthsc.edu
*Corresponding author: Yi Lu, Department of Pathology and Laboratory Medicine, University of Tennessee Health Science Center, Cancer Research Building, Room 258, 19 South Manassas Street, Memphis, TN 38163, USA, Email: ylu@uthsc.edu
Submission: May 12, 2020Published: June 02, 2020

ISSN:2637-773XVolume4 Issue5
Abstract
Toll-like receptors (TLRs) are single membrane-spanning proteins that are present on the membranes of immune cells involved in the innate response. Detection of pathogen-associated molecular patterns (PAMPs) initiates the adaptive immune response (antigen presentation). To date, 10 TLR genes have been determined in the human genome, and 13 in mice. TLRs are commonly adapted for use in neoplasia therapy, but different aspects of TLR function may suppress or promote tumor development. Connections between tumor development and inflammatory responses triggered by TLR activity have been elucidated, and TLR agonists are the subject of investigation for various cancer therapies. The purpose of this review is to focus on the relationship between TLR agonists and cancer and describe the underlying mechanisms that have been experimentally and clinically established about this relationship.
Keywords: TLRs; Toll-like receptors; Cancer therapy; Tumor development
Introduction
Table 1: TLRs in humans along with their respective agonists [57].
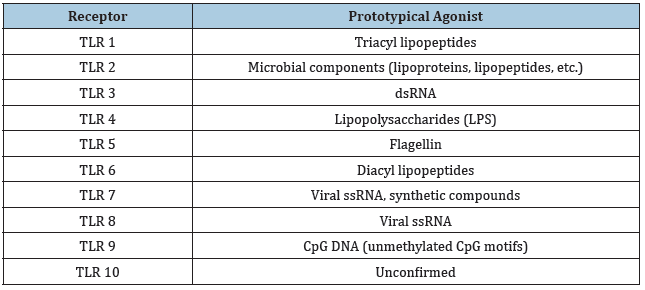
melanogaster, the Toll receptors play roles in embryological development and immunity, while TLRs function exclusively in the immune response in mammals [1]. At least five TLRs found in humans are homologous to their Drosophila counterparts, and all human TLRs result in the expression of nuclear factor-kappa B (NF-κB) [2,3]. The family of TLRs in humans are not all found in similar locations, as TLR genes span many chromosomes [4]. NF-κB leads to the activation of numerous cytokine genes, which promote an inflammatory response [4]. TLR10 is unique in that it is the only member of the TLR family to elicit an anti-inflammatory response, and TLR 11 technically exists in the human genome as a pseudogene [5,6]. Each TLR responds to different stimuli, and each stimulus is indicative of an invading pathogen, which can be detected both extracellularly and intracellularly [3,7-9] (Table 1 and Figure 1). Along with PAMPs, TLRs also detect damage-associated molecular patterns (DAMPs) or alarmins which are released endogenously by injured tissue cells which also result in an inflammatory exacerbation [7]. TLR genes have been found in more primitive life forms like the Caenorhabditis elegans, and this suggests that TLRs are an evolutionarily conserved mechanism of immunity [8]. For this reason, TLRs are known as a type of pattern recognition receptor (PRR) [9]. The characterization of TLRs as promoters of innate and adaptive immunity has furthered the understanding of TLR biology and makes way for investigation into their relationship with carcinogenesis.
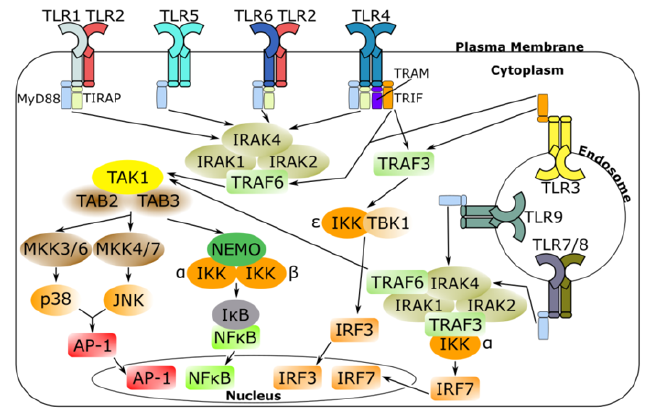
The role of TLRs in cancer lies within tumor development. Opposing data on the effects of TLRs exist, as there are TLR pathways that are proapoptotic, and those that are antiapoptotic. TLRs can stimulate cancer cell proliferation through NF-κB signaling, tissue repair mechanisms, and oxidative DNA damage [10]. While TLRs cannot be solely attributed to tumor activity in most cases, these pathways can be targeted in cancer therapy to control tumor growth (Figure 1). The expression of final gene products in the downstream pathways of TLRs contribute to their side-effect on tumors, and those effects will be discussed in this review.
Figure 1: Signal transduction pathways of human TLRs (1-10).
Structure of TLRs
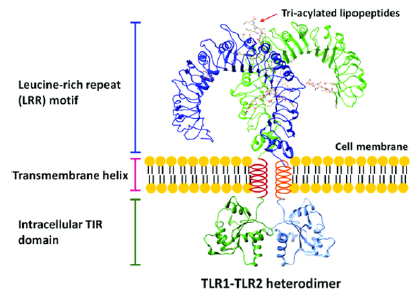
TLRs are classified as intrinsic, type I, single-pass receptors with a ligand binding site in the N-terminal domain and a signaling region in the C-terminal domain [11]. The signaling region consists of a Toll IL-1 receptor (TIR) that plays a crucial role in TLR signaling [11,12]. All TLRs possess a hydrophobic leucine-rich repeat (LRR) motif, which causes the receptor to curve into a horseshoe-like shape in the extracellular domain [11]. LRRs occur in sequences of 20-30 amino acids, and the leucine residues generate a hydrophobic core which is shielded from the hydrophilic side of the LRR that is saturated with solvent [12]. TLRs also contain several N-linked glycosylation sites, which vary in number along with the amount of LRRs across the different TLRs [11]. X-ray crystallography has contributed much to our current understanding of TLR structure at the molecular level [8].
TLR Signaling Pathways
TLRs dimerize into an “m” shape upon binding with a ligand [11]. For instance, TLR 2 is a heterodimer, associating with both TLR 1 and TLR 6 to detect lipopeptides) (Figure 2). Dimerization of TLRs attracts different adapter proteins depending on the dimer, such as myeloid differentiation primary response protein 88 (MyD88), TIR domain-containing adapter inducing IFNβ (TRIF), Trif-related adapter molecule (TRAM), and TIR domaincontaining adapter protein (TIRAP),and these receptors initiate the signal response, resulting in the transcription of interferons, cytokines, and chemokines [13]. Upon TLR activation, MyD88, the most common TLR adapter protein, interacts with the interleukin-1 receptor-associated kinase (IRAK) family of protein kinases, leading to the polyubiquitination of tumor necrosis factor receptor-associated factor 6 (TRAF 6) [13]. Polyubiquitin chains are required to activate transforming growth factor-beta-activated protein kinase 1 (TAK1) [14]. TAK1 may then phosphorylate two MAP kinase kinases (MKKs) to activate p38 (from MKK 3/6) and c-Jun N-terminal kinase (JNK) (from MKK 4/7) [13]. p28 and JNK activate the transcription factor for activator protein 1 (AP-1) [14]. The other NF-κB pathway results in the activation of the IKK complex by TAK1, which leads to the phosphorylation of NF-κB’s inhibitor, IκB, which is degraded by the 26S proteasome [13]. Both transcription factors exist outside of the nucleus in its inactive form and gain entry when activated [14]. The AP-1 and NF-κB pathways are used in almost all TLRs, which emphasizes the importance of the two genes in inflammatory signaling pathways.
Figure 2: TLR structure and dimerization represented by the TLR1-TLR2 heterodimer. Adapted via CC BY 4.0 from Ref. [20].
AP-1 and NF-κB transcription factors can also be promoted through the TRIF-dependent and MyD88-independent pathway [13]. TLRs 3 and 4 use this pathway [2]. TRIF also recruits TRAF3, which interacts with IκB kinase (IKK) and TANK-binding kinase 1 (TBK1) to stimulate the transcription of interferon regulatory factor 3 (IRF3) [2]. TLR4 is the only receptor to use both the MyD88 and TRIF pathway [2]. Therefore, it is crucial to control the activation of products through crosstalk between the two pathways. When TRAF3 is promoted in the pathway to produce IRF3, it also inhibits the MyD88 pathway, which balances the production of interferons and cytokines [14].
The overproduction of cytokines can be harmful to the body and there are regulatory molecules that exist to negatively modulate levels of cytokine production. Many studies into the regulation of TLR signaling has been done with TLR 4 and the LPS downstream pathways. IRAK-M has been shown to have defective kinase functionality and seems to block the TRAF6 complex from being formed in the MyD88 pathway, therefore lowering cytokine production [15]. An experiment showed that mice experienced higher levels of cytokine production when IRAK-M was removed during LPS exposure [16]. Suppressor of cytokine signaling 1 (SOCS1) negatively regulates the NF-κB pathway due to LPS exposure in macrophages [16]. β-arrestins, which play parts in G-protein regulation, also interfere with TRAF6 oligomerization, which prevents the polyubiquitination that is required to continue the NF-κB and AP-1 pathways [17]. Other protein pathways include negative regulation by A20, Lyp, BCAP/PI3K, Fliih, ST2, Triad3A, TRAF1, and IRAK-1c [17,18].
TIRAP and TRAM are adapter proteins that rarely exist alone downstream of a TLR dimer. TLR2 must have TIRAP in order to use the MyD88 pathway, which suggests that TLR2 is not fully compatible with signaling to MyD88 and that TIRAP acts as a link [14]. In TLR4 signaling, TRAM is required for the activation of TRIF, and TIRAP is required for MyD88 [2]. The MyD88-dependent and TRIF-dependent pathways make up the core of TLR signaling. The canonical pathway of NF-κB activation through TAK1 is shared by the two pathways, but each can promote different cellular responses outside of NF-κB. This marks their diversity and necessity for maintaining balance in the inflammatory response.
The Role of TLRs in Immunity
The basic principle of immunity is the distinction of self from non-self. To accomplish this distinction, innate and adaptive immunity work together to target pathogens. Innate immunity is found in both invertebrates and vertebrates and is known to be nonspecific in targeting pathogens, unlike antigen-primed B and T cells (adaptive immunity). TLRs are facilitators of innate immunity by mainly detecting PAMPs that indicate non-self, but not specifically targeting one epitope or pathogen. TLRs are generally found in immune cells of the innate response, namely macrophages and dendritic cells (DCs) [14]. When phagocytosis of a pathogen occurs in a macrophage, TLRs are gathered at the phagosome and react to certain constituents of the pathogen, which allows for a catered inflammatory response toward the pathogen [19]. It is important to note that TLRs simply detect foreign molecules and direct the response, but do not participate in it [20]. The success of phagosome maturation when engulfing pathogens has also been shown to be linked to the MyD88 pathway’s downstream activation of p38 [15].
TLR4, the most direct homolog to D. melanogaster’s Toll, is the most well-known and researched TLR. The discovery of LPS recognition by TLR4 began with the observation that mice with a mutation in the TLR4 gene were extremely unresponsive to LPS [21]. It recognizes LPS, which is found in Gram-negative bacteria and has been labeled as a prototypic initiator of the innate immune response [19]. Mutations in the TLR4 gene or a nonfunctional receptor can cause bacteria-driven septic shock due to faulty regulation of LPS [19].
TLR Mechanisms that Promote Tumor Growth
While TLR signaling pathways show promise in the field of cancer immunotherapy, they can also contribute to the proliferation of tumors. This contradictory side of TLR activity demonstrates the complexity of TLR interactions in the tumor microenvironment and how scientists have not been able to untangle all their mechanisms.
Inflammation
One facet of cancer development has linked tumor progression with inflammation. Inflammation is inherently an essential process when the body is injured or infected. However, the sustained presence of inflammatory molecules and growth factors resulting from inflammation enables tumor proliferation [22]. Various PRRs contribute to this inflammatory effect, including TLRs.
The activation of NF-κB, which can result from ligand binding to any human TLR, leads to elevated levels of an array of interleukins and cytokines [23]. Ninety percent of human tumors possess constitutively expressed NF-κB [23]. The accumulation of immune cells results in the release of anti-apoptotic factors and in turn, this promotes tumor survival [23]. Non-steroidal drugs have also been used to counteract the TLRs’ inflammatory effect along the NF-κB pathway in tumor therapy [23]. Immune cells also release various cytokines like vascular endothelial growth factor (VEGF) and growth factors like tumor necrosis factor alpha (TNFα) which induce tumor growth [24].
In breast cancers, TLR4 activation via LPS resulted in the increased expression of inflammatory regulators nitric oxide synthase2 (NOS2) and cyclooxygenase 2 (COX2), allowing for faster tumor growth due to the priming of the tumor microenvironment [25]. TLR4 along with other TLRs impair immune responses by DCs and T cells through the expression of factors like IL-12, IL-6, and NOS2. Bo Huang et al. [26] were able to show that tumor-possessing mice were able to survive longer when the TLR4 signaling cascade was inhibited.
Metabolic reprogramming
Tumor cells generally rely on glycolysis rather than oxidative phosphorylation (OXPHOS), otherwise known as the Warburg effect [27]. This switch is known as metabolic reprogramming and is a marker for cancer along with inflammation and immune response evasion [28]. The Warburg effect occurs in macrophages and DCs upon detection of TLR agonists, and this is also considered a marker for cancer [29].
TLR agonists can also activate the PI3K-AKT metabolic pathway in DCs which results in the expression of many glycolysis-related enzymes and glucose transporters, facilitating the switch from OXPHOS to glycolysis [30]. The pathway also inhibits AMP-activated protein kinase (AMPK), which plays a large role in controlling OXPHOS [30]. This reprogramming is accompanied by inflammation and the promotion of tumor survival [30].
Metastasis
TLR activity in myeloid cells has also been shown to promote metastatic potential [23]. TLRs 2,4, 5, and 9 have been shown to have this pro-metastatic effect due to a rise in secretion of inflammatory molecules. [31,32] Case by case studies have been done investigating each TLR in relation to various cancers, and a general trend of relationships can be inferred: TLRs are classic PRRs and their signaling cascades can result in the expression of many pro-inflammatory genes, which can create a favorable microenvironment for tumor proliferation and metastasis [32].
Usage of TLR Agonists as Adjuvants
Adjuvants are used to help vaccines provide a stronger, longerlasting immune response during treatment. Many TLR agonists that are effective as standard treatments can also be adjuvants in vaccinations for other diseases. TLR ligands have had a history of vaccine involvement with rabies, typhoid fever, influenza, pertussis, polio, and hepatitis A [33]. Along the same vein, previously established vaccines can be enhanced with TLR-related adjuvants, which is exciting for the further investigation into vaccine treatment in combination with TLR agonists [34-36].
Notable TLR Agonists in Cancer Therapy
BCG is an FDA-approved standalone treatment for bladder cancer, and it is derived from a reduced form of Mycobacterium bovisin a live form [37,38]. BCG has been observed to stimulate TLR 2,4, and 9 in bladder tumors, and acts as a tumor suppressive agonist and decreases the chance for recurrence. [37] TLR signaling initiated by BCG has been shown to increase TNFα, which stimulates the maturation of DCs, promoting the immune response to tumors [39]. Conversely, blocking of TLR 2 and 4 activity reduced the activation of DCs [40]. TLR 2 and TLR 4 play different roles in facilitating the response to BCG, where TLR2 induces the desired anti-tumor response for the therapy while TLR 4 assists in developing an immune response against BCG itself [41]. Natural killer cells (NKCs) also show more features of trained immunity and increased responsiveness upon BCG treatment [42].
The concept of bladder cancer treatment for BCG has been around for many decades. However, problems still exist today where BCG treatment fails or causes serious side effects. Since BCG’s precursor must be attenuated to reduce the effects of its toxicity as a bacterium, scientists have been looking towards optimizing the dosage of BCG treatment in order to control dangerous side effects [43]. A study done decreasing the full dose to a one-third dose was not able toestablish a connection between dosage and toxicity, and it seemed that reducing the dosage was negatively impacting the effectiveness of immunotherapy in higher-risk patients [43,44].
Imiquimod
Imiquimod is anFDA-approved treatment for basal cell carcinoma and genital warts and is derived from the imidazoquinoline family [38,45]. It is also undergoing many other clinical trials with various carcinomas and melanomas [46]. Imiquimod is a TLR7 agonist that results in anti-tumor activity by stimulating apoptosis through the mitochondrial pathway [47]. In basal cell carcinoma, imiquimod resulted in increased reactive oxygen species (ROS) production which induced p53-dependent apoptosis [48]. In TRAMP-C2 cells of prostate cancers, imiquimod was shown to arrest the cell cycle and inhibit further proliferation of tumors [49]. Imiquimod has been shown to have mixed effects on regulation of Tregs, by either inhibiting or promoting Treg number or function [50]. Imiquimod sensitizes cells in melanomas to radiotherapy and promotes autophagy-driven apoptosis in tumors [46].
Imiquimod was developed initially as a 5% topical cream for the treatment of mild skin disorders. When the antitumor effects of the treatment were discovered, many randomized studies have been undertaken in order to fully elucidate the therapeutic power of imiquimod with diseases involving the skin [51]. Imiquimod poses as a good candidate for synergistic treatment with other drugs. Its minimal side effects allow its antitumor potential to be complemented with immunostimulatory effects of other drugs, as treatment depends on TLR agonists also being able to stimulate the T cell response [51]. Other imidazoquinoline-derived treatments such as resiquimod and gardiquimodhave been investigated, andthey demonstrated the ability to target a wider breadth of cancers than imiquimod [47].
Monophosphoryl Lipid A (MPLA)
MPLA is an FDA-approved treatment for cervical carcinomas as an adjuvant of Cervarix [52]. MPLA is derived from Salmonella Minnesotalipo polysaccharides and acts as an agonist of TLR 2 and 4, although adjuvant treatment focuses on MPLA activation of TLR4 [37]. MPLA activates primarily the TRIF pathway and stimulates differentiation of memory CD8+ T cells as well as eliciting Th1 and Th2 responses [53,54].
The low toxicity of MPLA makes it a good choice as a vaccine adjuvant for humans. In a study on alpha-synuclein aggregation in Parkinson’s disease, MPLA provided a better alternative to LPS due to its less severe inflammatory side effects [55]. MPLA’s ability to retain immunological benefits of TLR signaling while reining in inflammatory responses makes it a prime option for further research and treatment. It is speculated that secretion of anti-inflammatory IL-10 hampers the effects of pro-inflammatory IFN γ and other molecules [54].
Polyinosinic: Polycytidylic Acid (Poly I:C)
Poly I: C is a synthetic analog to dsRNA, which makes it an effective agonist for TLR3 [56]. Poly I: C induces tumor cell apoptosis and T cell priming in colorectal and prostate cancers [57]. Poly I: C results in increased levels of antiapoptotic molecule Bcl-xL in activated CD4+ T cells, which promotes the cells’ survival [58]. The stimulation of TLR3 also leads to CD8+ T memory cell proliferation without the need for costimulation, which is significant when looking to inhibit tumorigenesis [38]. Poly I:C proves to be effective at inducing apoptosis in tumor cells in conjunction with 5-fluorouracil through a p53-based pathway and IFN- α, where a combination of all three treatments resulted in the highest apoptotic effect [59].
Ampligen (polyIC12U) and hiltonol (polyICLC) are other synthetic dsRNAs developed from poly I:C for use as adjuvants or in combination with other therapies [56,60]. Hiltonol is currently undergoing many clinical trials in order to evaluate its safety and efficacy in cancer vaccine treatments [61]. Poly I: C is also commonly used with CpG ODNs in order to treat tumors in mouse models [61].
CpG Oligodeoxynucleotide (CpG ODN)
CpG ODNs are short sequences of synthetic DNA that contain “CpG” motifs and are TLR 9 agonists [62]. Like poly I: C, CpG ODN can costimulate CD8+ T cells in vitro [63]. The TLR9 signaling pathway results in T Helper-1 response upregulation starting with a chemokine-releasing inflammatory stage followed by the adaptive response [64]. CpG ODNs by themselves do not pose an efficient antitumor treatment, but combinations with other treatments hold promise for cancer immunotherapies where immune stimulatory strategies can work alongside immune checkpoint regulation [65].
CpG ODNs inhibit tumor growth and arrested the cell cycle in the G1 phase in human glioma cells [66]. CpG ODNs interfere with tumorigenesis in renal cell carcinoma and induce caspase-driven apoptosis in neuroblastoma cells [57]. CpG ODNs show promise in conjunction with chemotherapy, driving tumor regression more strongly than chemotherapy alone in mouse models with colon carcinomas and non-small cell lung cancers [64]. 3M-052, a TLR 7/8 agonist, was administered in conjunction with CpG ODNs through intrapulmonary delivery, and the combined treatment alongside chemotherapy resulted in reduced tumor growth in metastatic lung cancer [67,68].
Current clinical trials
Table 2: TLRs and Their Specific Connections to Tumor Development in Cancers [68].
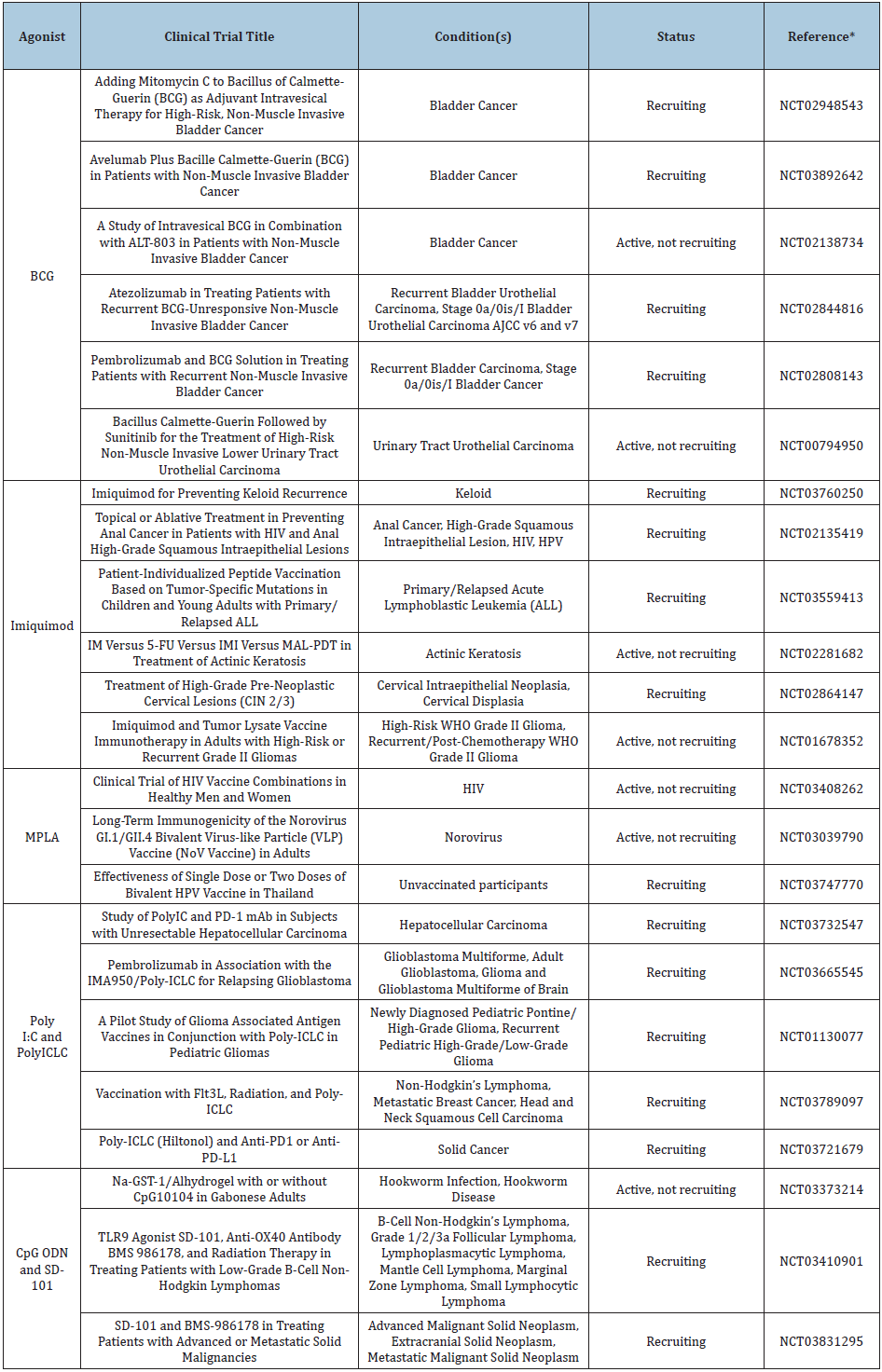
For each of the drugs mentioned above, a keyword search of the agonist was done on clinicaltrials.gov with a filter for recruiting, enrolling my invitation, and active, not recruiting statuses in interventional studies (clinical trials). Select trials involving specific use of these TLR agonists are cited in Table 2. Each clinical trial either involves direct therapy with the agonist or use of the agonist as an adjuvant.
Discussion
TLRs pose a compelling bridge between the immune system and the development of cancer. TLRs were once thought of as middlemen between the innate immune response and adaptive response, but now they play a much bigger role in cancer immunotherapy. Their ability to recognize general structures of pathogenic material presents the perfect opportunity to use experimental substances in immunotherapy. Currently, TLR agonist treatments are generally not consistent and effective enough to be used as a final therapy for a disease due to side effects of administration and immunoregulatory functions that prevent an over clocked immune response. To address the latter, TLR agonists can be administered in tandem with substances that counteract immunoregulatory functions like Treg cells. This combinatorial treatment strategy is the subject of many ongoing clinical trials.
A current hurdle in TLR agonist treatments is the contradictory effects that are achieved in some clinical trials. TLRs seem to elicit pro-tumor and anti-tumor effects, which is due to the presence of TLRs on tumor cells and immune cells. This can likely be overcome as more is known about TLR signaling pathways and ways to target mediator molecules are discovered.
An appreciable amount of research still must be conducted to fully understand the mechanisms of TLR signaling, as various ligands result in different downstream responses, and the key to TLR immunotherapy is correctly modulating these responses. As clinical trials seek to increase the immunospecificity and potential of TLR agonist activity, new treatments will arise that are feasible in implementation for their anti-tumor effects.
Competing Interests
The authors have declared that no competing interest exists.
References
- Valanne S, Wang JH, Rämet M (2011) The drosophila toll signaling pathway. The Journal of Immunology 186(2): 649-656.
- Kawai T, Akira Shizuo (2007) Signaling to NF-κB by Toll-like receptors. Trends in Molecular Medicine 13(11): 460-469.
- Rock FL, Hardiman G, Timans JC, Kastelein RA, Bazan JF(1998) A family of human receptors structurally related to Drosophila Toll. Proc Natl Acad Sci USA 95(2): 588-593.
- HUGO Gene Nomenclature Committee. Gene group: Toll like receptors (TLR).
- Oosting M, Cheng SC, Bolscher JM, Vestering-Stenger R, Plantinga TS, et al.(2014) Human TLR10 is an anti-inflammatory pattern-recognition receptor. Proc Natl Acad Sci USA 111(42): 4478-4484.
- Gazzinelli RT, Medonca-Neto R, Lilue J, Howard J, Sher A (2014) Innate resistance against Toxoplasma Gondii: An evolutionary tale of mice, cats, and men. Host & Microbe 15(2): 132-138.
- McCarthy CG, Goulopoulou S, Wensceslau CF, Spitler K, Matsumoto T, et al. (2014) Toll-like receptors and damage-associated molecular patterns: novel links between inflammation and hypertension. Heart and Circulatory Physiology 306(2): 184-196.
- Christmas P (2010) Toll-like receptors: Sensors that detect infection. Nature Education 3(9): 85.
- Satoh T, Akira S (2016) Toll-like receptor signaling and its inducible proteins. Microbiology Spectrum 4(6).
- Rakoff-Nahoum S, Medzhitov R (2009) Toll-like receptors and cancer. Nature Reviews: Cancer 9: 57-63.
- Botos I, Segal DM, Davies DR (2011) The structural biology of Toll-like receptors. Structure 19(4): 447-459.
- Jin MS, Lee JO (2008) Structures of the Toll-like receptor family and its ligand complexes. Immunity 29(2): 182-191.
- Kawai T, Akira S (2006) TLR signaling. Cell Death and Differentiation 13: 816-825.
- Kawasaki T, Kawai T (2014) Toll-like receptor signaling pathways. Frontiers in Immunology 5: 461.
- Takeda S, Akira S (2005) Toll-like receptors in innate immunity. International Immunology 17(1): 1-14.
- Kobayashi K, Hernandez LD, Galán JE, Janeway CA, Medzhitov R, et al. (2002) IRAK-M is a negative regulator of toll-like receptor signaling. Cell 110(2): 191-202.
- Miggin SM, O’Neill LAJ (2006) New insights into the regulation of TLR signaling. Journal of Leukocyte Biology 80(2): 220-226.
- Hamerman JA, Pottle J, Ni M, He Y, Zhang ZY, et al. (2016) Negative regulation of TLR signaling in myeloid cells-Implications for autoimmune diseases. Immunological Reviews 269(1): 212-227.
- Aderem A, Ulevitch R (2000) Toll-like receptors in the induction of the innate immune response. Nature 406(6797): 782-787.
- Gao W, Xiong Y, Li Q, Yang H (2017) Inhibition of toll-like receptor signaling as a promising therapy for inflammatory diseases: A journey from molecular to nano therapeutics. Frontiers in Physiology 8: 508.
- Qureshi ST, Larivière L, Leveque G, Clermont S, Moore KJ, et al. (1999) Endotoxin-tolerant Mice Have Mutations in Toll-like Receptor 4 (Tlr4). Journal of Experimental Medicine 189(4): 615-625.
- Coussens LM, Werb Z (2002) Inflammation and cancer. Nature 420: 860-867.
- Shcheblyakov DV, Logunov DY, Tukhvatulin AI, Shmarov MM, Naroditsky BS, et al. (2010) Toll-like receptors (TLRs): The role in tumor progression. Acta Naturae 2(3): 21-29.
- Braunstein MJ, Kucharczyk J, Adams S (2018) Targeting Toll-like receptors for cancer therapy. Targeted Oncology 13(5): 583-598.
- Ridnour LA, Cheng RYS, Switzer CH, Heinecke JL, Ambs S, et al. (2013) Molecular pathways: Toll-like receptors in the tumor microenvironment-poor prognosis or new therapeutic opportunity. Clinical Cancer Research 19(6): 1340-1346.
- Huang B, Zhao J, Li H, He KL, Chen Y, et al. (2005) Toll-like receptors on tumor cells facilitate evasion of immune surveillance. Cancer Research 65(12): 5009-5014.
- Alfarouk KO, Verduzco D, Rauch C, Muddathir AK, Adil HHB, et al. (2014) Glycolysis, tumor metabolism, cancer growth, and dissemination. A new pH-based etiopathogenic perspective and therapeutic approach to an old cancer question. Oncoscience 1(12): 777-802.
- Yoshida GJ (2015) Metabolic reprogramming: the emerging concept and associated therapeutic strategies. Journal of Experimental & Clinical Cancer Research 34: 111.
- Huang L, Xu H, Peng G (2018) TLR-mediated metabolic reprogramming in the tumor microenvironment: potential novel strategies for cancer immunotherapy. Cellular & Molecular Immunology 15: 428-437.
- Biswas S (2015) Metabolic reprogramming of immune cells in cancer progression. Immunity 43(3): 435-449.
- Li K, Qu S, Chen X, Wu Q, Shi M (2017) Promising targets for cancer immunotherapy: TLRs, RLRs, and STING-mediated innate immune pathways. International Journal of Molecular Sciences 18(2): 404.
- Song EJ, Kang MJ, Kim YS, Kim SM, Lee SE, et al.(2011) Flagellin promotes the proliferation of gastric cancer cells via the Toll-like receptor 5. International Journal of Molecular Medicine 28(1): 115-119.
- Duthie MS, Windish HP, Fox CB, Reed SG (2011) Use of defined TLR ligands as adjuvants within human vaccines. Immunological Reviews 239(1): 178-196.
- Dowling DJ (2018) Recent advances in the discovery and delivery of TLR7/8 agonists as vaccine adjuvants. Immuno Horizons 2(6): 185-197.
- Dowling JK, Mansell A (2016) Toll-like receptors: the swiss army knife of immunity and vaccine development. Translational Immunology 5(5): e85.
- Onodera T, Hosono A, Odagiri T, Tashiro M, Kaminogawa S, et al. (2016) Whole-virion influenza vaccine recalls an early burst of high-affinity memory B cell response through TLR signaling. The Journal of Immunology 196(10): 4172-4184.
- Smith M, García-Martínez E, Pitter MR, Fucikova J, Spisek R, et al. (2018) Trial watch: Toll-like receptor agonists in cancer immunotherapy. OncoImmunology 7(12): e1526250.
- Kaczanowska S, Joseph AM, Davila E (2013) TLR agonists: our best frenemy in cancer immunotherapy. Journal of Leukocyte Biology 93(6): 847-863.
- Tsuji S, Matsumoto M, Takeuchi O, Akira S, Asuma I, et al. (2000) Maturation of human dendritic cells by cell wall skeleton of mycobacterium bovis bacillus calmette-guérin: Involvement of toll-like receptors. Infection and Immunity 68(12): 6883-6890.
- Uehori J, Matsumoto M, Tsuji S, Akazawa T, Takeuchi O, et al. (2003) Simultaneous blocking of human toll-like receptors 2 and 4 suppresses myeloid dendritic cell activation induced by mycobacterium bovis bacillus calmette-guérin peptidoglycan. Infection and Immunity 71(8): 4238-4249.
- Heldwin KA, Liang MD, Andresen TK, Thomas KE, Marty AM, et al. (2003) TLR2 and TLR4 serve distinct roles in the host immune response against Mycobacterium bovis BCG. Journal of Leukocyte Biology 74(2): 277-286.
- Kleinnijenhuis J, Quintin J, Preijers F, Joosten LAB, Jacobs C, et al. (2014) BCG-induced trained immunity in NK cells: Role for non-specific protection to infection. Clinical Immunology 155(2): 213-219.
- Fuge O, Vasdev N, Allchorne P, Green JSA (2015) Immunotherapy for bladder cancer. Research and Reports in Urology 7: 65-79.
- Brausi M, Oddens J, Sylvester R, Bono A, van de Beek C, et al. (2014) Side Effects of Bacillus Calmette-Guérin (BCG) in the treatment of intermediate- and High-risk Ta, T1 papillary carcinoma of the bladder: Results of the EORTC Genito-Urinary Cancers group randomised phase 3 study comparing one-third dose with full dose and 1 year with 3 years of maintenance BCG. European Urology 65(1): 69-76.
- Ma F, Zhang J, Zhang J, Zhang C (2010) The TLR7 agonists imiquimod and gardiquimod improve DC-based immunotherapy for melanoma in mice. Cellular & Molecular Immunology 7(5): 381-388.
- Cho J, Lee HJ, Ko HJ, Yoon BI, Choe J, et al. (2017) The TLR7 agonist imiquimod induces anti-cancer effects via autophagic cell death and enhances anti-tumoral and systemic immunity during radiotherapy for melanoma. Oncotarget 8(15): 24932-24948.
- Chi H, Li C, Zhao FS, Zhang L, Ng TB, et al. (2017) Anti-tumor activity of Toll-like receptor 7 agonists. Frontiers in Pharmacology 8: 304.
- Huang SW, Chang SH, Mu SW, Jiang HY, Wang ST, et al. (2016) Imiquimod activates p53-dependent apoptosis in a human basal cell carcinoma cell line. Journal of Dermatological Science 81(3): 182-191.
- Han JH, Lee J, Jeon SJ, Choi ES, Cho SD, et al. (2013) In vitro and in vivo growth inhibition of prostate cancer by the small molecule imiquimod. International Journal of Oncology 42(6): 2087-2093.
- Lu H (2014) TLR agonists for cancer immunotherapy: tipping the balance between the immune stimulatory and inhibitory effects. Frontiers in Immunology 5: 83.
- Vacchelli E, Galluzzi L, Eggermont A, Fridman WH, Galon J, et al. (2012) Trial watch: FDA-approved Toll-like receptor agonists for cancer therapy. Oncoimmunology 1(6): 894-907.
- Gregg JA, Harberts E, Gardner FM, Pelletier MR, Cayatte C, et al. (2017) Rationally designed TLR4 ligands for vaccine adjuvant discovery. mBio 8(3): e00492-17.
- Cui W, Joshi NS, Liu Y, Meng H, Kleinstein SH, et al. (2014) TLR4 ligands LPS and MPLA differentially regulate effector and memory Cd8+ T cell differentiation. The Journal of Immunology 192(9): 4221-4232.
- Casella CR, Mitchell TC (2008) Putting endotoxin to work for us: monophosphoryl lipid A as a safe and effective vaccine adjuvant. Cellular and Molecular Life Sciences 65(20): 3231-3240.
- Venezia S, Refolo V, Polissidis A, Stefanis L, Wenning GK, et al. (2017) Toll-like receptor 4 stimulation with monophosphoryl lipid A ameliorates moto deficits and nigral neurodegeneration triggered by extraneuronal α-synucleoinopathy. Molecular Neurodegeneration 12(1): 52.
- Li JK, Balic J, Yu L, Jenkins B (2017) TLR agonists as adjuvants for cancer vaccines. Advances in Experimental Medicine and Biology 1024: 195-212.
- Du B, Jiang QL, Cleveland J, Liu BR, Zhang D(2016) Targeting Toll-like receptors against cancer. J Cancer Metastasis Treat 2: 463-470.
- Gelmen AE, Zhang J, Choi Y, Turka LA (2004) Toll-like receptor ligands directly promote activated CD4+ T cell survival. The Journal of Immunology 172(10): 6065-6073.
- Taura M, Fukuda R, Suico MA, Eguma A, Koga T, et al. (2010) TLR3 induction by anticancer drugs potentiates poly I:C-induced tumor cell apoptosis. Cancer Sci 101(7): 1610-1617.
- Sharma S, Zhu L, Davoodi M, White MH, Lee JM, et al. (2013) TLR3 agonists and proinflammatory antitumor activities. Expert Opinion on Therapeutic Targets 17(5): 481-483.
- Ammi R, Waele JD, Willemen Y, Brussel IV, Schrijvers DM, et al. (2015) Poly(I:C) as cancer vaccine adjuvant: Knocking on the door of medical breakthroughs. Pharmacology & Therapeutics 146: 120-131.
- CpG Oligodeoxynucleotide.
- Bendigs S, Salzer U, Lipford GB, Wagner H, Heeg K (2006) CpG-oligodeoxynucleotides co-stimulate primary T cells in the absence of antigen-presenting cells. European Journal of Immunology 29(4): 1209-1218.
- Murad YM, Clay TM (2009) CpG Oligodeoxynucleotides as TLR9 Agonists. Bio Drugs 23(6): 361-375.
- Adamus T, Kortylewski M (2018) The revival of CpG oligonucleotide-based cancer immunotherapies. Contemporary Oncology 22(1A): 56-60.
- Li X, Liu D, Liu X, Jiang W, Zhou W, et al. (2012) CpG ODN107 potentiates radiosensitivity of human glioma cells via TLR9-mediated NF-κB activation and NO production. Tumor Biology 33(5): 1607-1618.
- Goguet E, Klinman DM, Tross D (2018) Intrapulmonary delivery of TLR agonists associated with systemic chemotherapy to treat metastatic cancer. The Journal of Immunology 200(1).
- gov.
© 2020. Yi Lu. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






