- Submissions

Full Text
Novel Approaches in Cancer Study
The Role of “Hedgehog Signaling” in AML
Pavan Kumar Dhanyamraju1*, Yevgeniya Bamme1, Dhimant Desai2, and Sinisa Dovat1
1Department of Pediatrics, Pennsylvania State University College of Medicine, Hershey, PA 17033, USA
2 Department of Pharmacology, Pennsylvania State University College of Medicine, Hershey, PA 17033, USA
*Corresponding author: Pavan Kumar Dhanyamraju, Department of Pediatrics, Pennsylvania State University College of Medicine,Hershey-17033, PA, USA, Tel: 717-531-1841, Email: pdhanyamraju@pennstatehealth.psu.edu
Submission: March 18, 2020Published: April 06, 2020

ISSN:2637-773XVolume4 Issue3
Abstract
The Hedgehog (Hh) signal transduction pathway is involved in diverse physiological processes such as cell survival, proliferation, differentiation, embryonic development, and fetal determination. Perturbations in this pathway leading to its aberrant activation have been implicated in various cancers including acute myeloid leukemia (AML). AML is a highly complex hematological malignancy resulting due to accumulation of abnormal myeloblasts in the bone marrow leading to bone marrow failure and ultimately death. Recently, the role of hedgehog signaling in AML pathogenesis has been uncovered and thus pharmacological targeting of the hedgehog pathway in AML has become a prime therapeutic option. In this mini-review, we highlight the role of the hedgehog pathway in AML and review the latest drugs as well as possible Hh targets in treating AML.
Keywords: Hedgehog signaling; Smoothened; Patched; Acute myeloid leukemia; Targets; Drugs; Treatments; Canonical pathway; Noncanonical pathway
Abbreviations: Hedgehog (Hh), Acute Myeloid Leukemia (AML), Desert Hedgehog (DHH), Indian Hedgehog (IHH), GLI1(Glioma-Associated Oncogene Homolog 1), Sonic Hedgehog (SHH), Primary Cilium (PC), Patched (PTCH), Smoothened (SMO), Rhabdomyosarcoma (RMS), Basal Cell Carcinoma (BCC), Adverse Events (AEs), Receptor Tyrosine Kinases (RTKs), Cancer Stem Cells (CSC), Leukemic Stem Cells (LSC), Food and Drug Administration (FDA), Hematopoietic Stem Cell Transplantation (HSCT), Minimal Residual Disease (MRD), UDP-Glucuronosyl Transferase (UGT1A), Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR), GLI Antagonist (GANT61), Bone Marrow (BM)
Introduction
The development of multicellular organisms is a precisely controlled and highly orchestrated process that depends on the regulation of signal transduction pathways in a spatial, as well as, context-based manner. The importance of controlled activation as well as timely termination of signals is critical for the ideal functioning of organisms [1]. The hedgehog signaling pathway is one such pathway that plays a quintessential role in mediating several fundamental processes including cell survival, differentiation, proliferation, regeneration, tissue polarity, patterning, fetal determination, and maintenance of stem cells [2,3]. Hyperactivation of Hh signaling has been shown to cause a variety of cancers like medulloblastoma, rhabdomyosarcoma (RMS), basal cell carcinoma (BCC), melanoma, lung, gastric, breast, prostate, colorectal, and pancreatic cancers [4].
The Hedgehog (Hh)gene was discovered 40 years ago by the pioneering work of Eric F. Weischaus and Christiane Nusslein-Volhard as a “segment-polarity” gene that controlled patterning in Drosophila embryonic cuticle [5]. One of the basic requirements for the Hh pathway is the Hh ligands, which initiate hedgehog signal transmission. For normal Hh signaling to occur the production, secretion, processing, and transportation of these ligands at appropriate levels is required. In mammalians, Desert Hedgehog (DHH), Indian Hedgehog (IHH), and Sonic Hedgehog (SHH) are the three kinds of Hh ligands found out, of which only SHH has been extensively studied [6]. These Hh ligands bind to a 12-span trans membrane receptor known as Patched (PTCH), which acts as a negative regulator of the pathway. Ptch localizes to primary cilium (PC) in the absence of Hh ligands and represses7-transmembrane protein Smoothened (SMO), a positive regulator of the pathway, by inhibiting its entry into the PC and thereby shutting down the canonical hedgehog signaling pathway. Whereas, in the presence of Hh ligands, PTCH is displaced from PC leading to the derepression of SMO by inducing a conformational change. This results in the activation of downstream signaling leading to transcription of target genes like GLI1, PTCH1, PTCH2, HHIP1, BCL2, FOXF1, FOXL1, JAG2, MYCN, CCND1, CCND2, CFLAR, PRDM1, Follistatin, and GREM1 [7]. One of the important negative regulators of the canonical Hh pathway is the Suppressor of Fused (SUFU) which is involved in inhibiting the transcription of Hh target genes. Apart from the canonical Hh pathway (HH-PTCHSMO- dependent), non canonical Hh signaling (SMO-independent activation of GLI) pathways have also been reported which are implicated in the activation of GLI genes as well as involved in post-translational modifications of GLI proteins. Together, the components of canonical and noncanonical Hh signaling pathways are a complex network of signaling modules regulating myriad facets of cellular behavior.
Acute myeloid leukemia (AML) is a complex, aggressive, heterogeneous hematological malignancy. It arises due to abnormal myeloblast accumulation in the bone marrow and peripheral blood. If untreated, it often leads to death. Radiotherapy, chemotherapy and hematopoietic stem cell transplantation (HSCT) are widely employed as therapeutic strategies for AML treatment. Despite outstanding progress in the field of drug development and AML biology, the overall 5-year survival rates are relatively low (~28.3%). Therefore, there is a need for novel treatment modalities. Several signaling cascades like PI3K/AKT, RAF/MEK/ERK and receptor tyrosine kinases (RTKs) are deregulated in AML [8]. Furthermore, growing evidence shows that early developmental pathways like Hh signaling play an important role in AML pathogenesis.
Hh signaling cascade is crucial for the function and maintenance of leukemic stem cells (LSC) or cancer stem cells (CSC). These, LSCs/CSCs are resistant to a wide variety of chemotherapeutic drugs and usually give rise to minimal residual disease (MRD), and eventually relapse to full-blown disease [9]. Zahreddine et al. [10] demonstrated that the Hh pathway contributes to Ara-c and ribavirin drug resistance in AML cells. Elevated levels of UDPglucuronosyl transferase (UGT1A) and GLI1enzyme were noticed in AML blast cells. Further, an association between UGT1A activity and elevated GLI1 levels was observed, which leads to a poor prognosis. UGT1As are involved in the glucuronidation of several drugs thereby altering their metabolic and functional activity. In this case, they used GDC-0449 (Vismodegib) to inhibit the Hh pathway. In conclusion, the data shows that resistance to Ara-c and ribavirin can be circumvented by using inhibitors against GLI1.
In a recent study, two independent AML patient cohorts were used to study the expression of key components of the Hh pathway. Both GLI1 and GLI2 transcription factors were found to be elevated in AML patients. Also, a clear association between the expression levels of GLI2 and the mutational status of FLT3 was noticed. A paracrine mode of Hh signaling was described to be active in AML cells as no Hh ligands could be detected by quantitative real-time polymerase chain reaction (qRT-PCR). Furthermore, the authors observed elevated levels of DHH in the serum of the patients indicating that it was shed into the bloodstream by the bone marrow (BM) microenvironment and not by the AML cells. The antileukemic potential of Hh pathway inhibition was also demonstrated in-vivo and in-vitro by shRNA (GLI1 and GLI2) and via GLI inhibition (GANT61). Taken together, pharmacological GLI inhibition could be an effective therapeutic strategy to treat AML patients [11].
Li et al. [12] showed that in radiation-resistant HL-60 cells (HL60/rx) elevated GLI1 and SMO levels were observed in comparison to radiation-sensitive cells, implicating the role of active Hh signaling in resistance to radiotherapy. Combination therapy using Hh signaling inhibitors along with radiotherapy can be a promising therapeutic strategy in such cases. In another study by Fukushima et al. [13] the role of selective smoothened inhibitor PF- 04449913 (Glasdegib) in AML cells was demonstrated. Increased Hh signaling activity was observedin CD34+ cells to that of CD34- cells and treatment with PF-04449913 led to a reduction in the quiescent cell population. Moreover, in a serial transplantation mouse model, PF-04449913 treatment led to a decrease in the leukemia-initiating potential of AML cells. Further, treatment of AML cells with PF- 04449913 led to sensitivity of AML cells to cytosine arabinoside. Altogether, the data indicate that pharmacological inhibition of SMO could be a useful treatment option for AML patients. Several other studies implicating Hh signaling pathway in the pathogenesis of AML have been shown but mentioning all the studies is beyond the scope of this mini-review.
Conclusion and Future Perspective
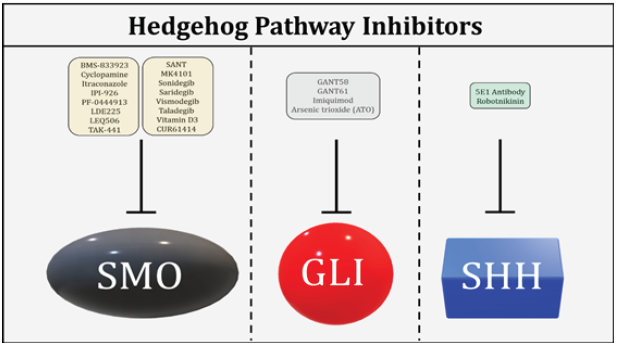
The role of Hh signaling pathway in the pathobiology of myriad cancers including AML has been a major area of research for almost a decade now. With recent advances in the field of molecular biology, a much clearer picture of the Hh signaling pathway in AML has emerged. As our understanding with regards to various components of the canonical and non-canonical Hh signaling has improved, it has now become possible to target these components with novel drugs. Several Hh inhibitors targeting different components of Hh signaling have been developed see (Figure 1) but only a few have been approved by the Food and Drug Administration (FDA) for therapeutic use due to safety issues. A range of adverse events (AEs) have been observed with Hh inhibitors, the most common being alopecia and gastrointestinal problems. Thus, there is a need to develop much safer and more effective drugs to treat various cancers including AML where aberrant Hh signaling plays a prominent role. Furthermore, the genomic revolution has opened a new field of personalized medicine to reduce AEs and improve treatment outcomes for patients. Finally, our search for answers has just started and more needs to be known and explored. Through advances in science and medicine, we are on track to develop better treatment options to treat malignancies like AML.
Figure 1: Hh pathway inhibitors targeting key Hh components i.e SMO, GLI and SHH.
Acknowledgment
Penn State Cancer Institute and Four Diamonds Fund of Pennsylvania State University College of Medicine, Hershey, PA -17033, USA.
Author Contributions
Conceptualization, manuscript original draft writing, editing PK, manuscript review YB, DD, and S.D. All authors have read and agreed to the published version of the manuscript.
Conflict of interest
The authors declare no conflict of interest.
References
- Teperino R, Aberger F, Esterbauer H, Riobo N, Pospisilik JA (2014) Canonical and non-canonical hedgehog signalling and the control of metabolism. Semin Cell Dev Biol 33: 81-3392.
- Ingham PW, Nakano Y, Seger C (2011) Mechanisms and functions of Hedgehog signalling across the metazoa. Nat Rev Genet 12(6): 393-406.
- Terao T, Minami Y (2019) Targeting Hedgehog (Hh) Pathway for the acute myeloid leukemia treatment. Cells 8(4): 312.
- Skoda AM, Simovic D, Karin V, Kardum V, Vranic S, et al. (2018) The role of the Hedgehog signaling pathway in cancer : A comprehensive review. Bosn J Basic Med Sci 18(1): 8-20.
- Sari IN, Thi L, Phi H, Jun N, Wijaya YT, et al. (2018) Hedgehog signaling in cancer : A prospective therapeutic target for eradicating cancer stem cells. Cells 7(11): 208.
- Varjosalo M, Taipale J (2008) Hedgehog: Functions and mechanisms. Genes and Development 22(18): 2454-2472.
- Katoh Y, Katoh M (2009) Hedgehog target genes: Mechanisms of carcinogenesis induced by aberrant hedgehog signaling activation. Curr Mol Med 9(7): 873-886.
- Scholl C, Gilliland DG, Fröhling S (2008) Deregulation of signaling pathways in acute myeloid leukemia. Semin Oncol 35(4): 33-345.
- Aberger F, Hutterer E, Sternberg C, Del Burgo PJ, Hartmann TN (2017) Acute myeloid leukemia - strategies and challenges for targeting oncogenic Hedgehog/GLI signaling. Cell Commun Signal 15(1): 8.
- Zahreddine HA, Culjkovic-Kraljacic B, Assouline S, Gendron P, Romeo AA, et al. (2014) The sonic hedgehog factor GLI1 imparts drug resistance through inducible glucuronidation. Nature 511(7507): 90-93.
- Wellbrock J, Latuske E, Kohler J, Wagner K, Stamm H, et al. (2015) Expression of hedgehog pathway mediator GLI represents a negative prognostic marker in human acute myeloid leukemia and its inhibition exerts Antileukemic effects. Clin Cancer Res 21(10): 2388-2398.
- Li X, Chen F, Zhu Q, Ding B, Zhong Q, et al. (2016) Gli-1/PI3K/AKT/NF-kB pathway mediates resistance to radiation and is a target for reversion of responses in refractory acute myeloid leukemia cells. Oncotarget 7(22): 33004-33015.
- Fukushima N, Minami Y, Kakiuchi S, Kuwatsuka Y, Hayakawa F, et al. (2016) Small-molecule Hedgehog inhibitor attenuates the leukemia-initiation potential of acute myeloid leukemia cells. Cancer Sci 107(10):1422-1429.
© 2020. Pavan Kumar Dhanyamraju. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






