- Submissions

Full Text
Novel Approaches in Cancer Study
Skeletal Related Events in Metastasis Breast Cancer: Case Report
Ayman Rasmy1,2* and Amal Ameen3
1 Oncology Department, Zagazig University, Zagazig, Egypt
2 Oncology Department, King Saud Medical City, Riyadh-KSA
3 Microbiology Department, Faculty of Medicine, Fayoum University, Egypt
*Corresponding author:Ayman Rasmy MD, Medical Oncology, Zagazig University, Zagazig-Egypt
Submission: January 21, 2019;Published: February 11, 2019

ISSN:2637-773XVolume2 Issue2
Introduction
Breast cancer is the most common malignancy distressing females in the world. The bone metastases are one of the most common site of disease dissemination. Up to 75% of stage IV Breast cancer patients developing skeletal metastases [1] metastatic bone disease can result in skeletal related events (SREs), including humoral hypercalcaemia of malignancy (HHM), pathological fractures, spinal cord compression, and pain [2]. The median time from bone metastases diagnosis to first SRE can be as short as 1.8 months, with the incidence of SREs growing considerably during the first 12 months following diagnosis [3]. This Bone metastases affect patient survival, movement, and quality of life by negative impact. The molecular mechanisms involved in the metastasis, colonization, and proliferation of breast cancer cells in bone are multifaceted and include crosstalk between breast cancer cells and the bone microenvironment. The capability of metastatic breast cancer cells to capture normal biological processes involved in bone remodeling is a key driver of osteolytic and osteoblastic bone lesions. As such, our understanding of how breast cancer cells manipulate normal bone remodeling pathways is essential for the development of new therapeutic agents to improve patient outcomes.
Case Report
Forty-one years old Female patient, previously medically free, for 4 months start to complain of back pain and left breast lump, pain increased gradually until pt. become unable to pear her weight., she had medical advice outside with only with analgesic prescription for pain. At that time, she had painless breast mass which increased gradually, no nipple discharge, no real or observed disfiguring of breast, recently, she presents to emergency with severe back pain unable to walk or stand. CT spine with contrast done initially found to have multilevel spinal cord compression. During admission she found to have hypercalcemia treated with Calcitonin & Denosemab.
Social history
She is married, lactating mother of one child 2months ago
Family history
No family history of similar condition
Systemic examination
Clinically she conscious, oriented, lying flat in the bed, performance ECOG (3-4) since admission due to back pain Neurology examination showed she had lower limb weakness with power grade 3, upper limb no weakness with constipation and difficulty in urination with catheter. Other system normal examination. Tru- Cut Biopsy was done with pathology full assessment revealed that it diagnosed as IDC, grade II. ER: positive, strong intensity, 80% of tumor cells. PR: negative., HER2: equivocal (2+), sent for FISH. ki- 67: less than 5%.
Radiology work up
i. CT spine Findings suggestive of diffuse osseous metastatic lesion.
a) Diffuse destructive changes in the spine with reduced vertebral heights more at T5, and less in T2, T4- T10, L1, L3 and L5 represent multilevel compression fractures.
b) Narrowing the spinal canal at level T5 and T6. Also, the posterior element is involved multiple osteolytic lesion in the visualized sternum and ribs.
c) Pelvic bones and femora are involved.
ii. MRI spine: Diffuse bony metastasis
iii. U/S breast: Suspicious left breast 12 o’clock position mass with abnormal left axillary lymph nodes. BIRADS 5
iv. CT CAP: Multiple osteolytic and osteoblastic lesions seen in the lumbar and visualized lower dorsal vertebrae with iliac bone involvement and visualized femora. Evidence of motheaten lesions seen in the liver parenchyma mainly involving the left lobe segment four and three with focal area involving the segment 6 and 8. There are multiple bony metastasis involving the thoracic spine as described. Bilateral centri acinar emphysematous changes without any significant focal lesion or metastasis noted
v. KUB U/S: Both kidneys are normal in size but increased echogenicity grade 1-2 picture of chronic medical renal disease, left renal stone=1 cm is seen, no hydronephrosis.
Currently patient will start on urgent radiotherapy then will start on systemic treatment as per stander guidline.
Pathogenesis of breast cancer metastases to bone
Figure 1:

Invasion of BC cells into bone deregulates normal bone remodelling such that bone resorption and formation become uncoupled, resulting in pathological bone loss (osteolysis) or bone formation (osteoblastic lesions). Metastasis of BC cells to bone gives rise to osteolytic, osteoblastic and mixed lesions, typically with sclerotic and lytic processes occurring concurrently in affected bone [4]. The ability of BC cells to produce osteomimetic factors facilitates BC cell survival and colonisation within the bone microenvironment and promotes the development of bone metastases (Figure 1).
Management of metastatic bone disease of breast cancer
Figure 2:
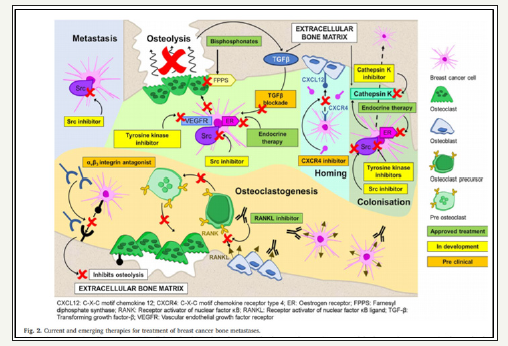
Systemic treatments including endocrine therapy, chemotherapy and targeted therapies such as anti-HER2 drugs, mTOR inhibitor, CDK 4/6 inhibitor) can be effective in patients to reduce the burden of bone disease and other disease sites. Glucocorticoids which are commonly used in the symptomatic management of patients with metastatic BC, enhance osteoclasto genesis and reduce osteoclast genesis. Glucocorticoids inhibit OPG and activate RANKL synthesis by pre-osteoblasts to increase osteoclast genesis and stimulate bone resorption [5]. Upon binding of glucocorticoids to glucocorticoid receptors, the receptors tether to the AP-1 site of the interleukin- 11 (Il-11) promotor to inhibit Il-11 production, which in turn inhibits osteoblast genesis [6].
There are multiple treating agents currently presented for the managing of metastatic breast cancer to bone by bisphosphonates and RANK/RANKL inhibitors. Numerous novel therapeutic targets are also emerging demonstrating potential clinical utility in the treatment of BC bone metastases, including cathepsin K inhibitors, Src inhibitors, TGF-β blockade, CXCR4 inhibitors, and αvβ3 integrin antagonists (Figure 2).
References
- Kuchuk I, Hutton B, Moretto P, Ng T, Addison CL, et al. (2013) Incidence: consequences and treatment of bone metastases in breast cancer patients-experience from a single cancer centre. Journal of Bone Oncology 2(4): 137-144.
- Cleeland C, Von MR, Walker MS, Wang Y, Gao J, et al. (2016) Burden of symptoms associated with development of metastatic bone disease in patients with breast cancer. Care Cancer 24(8): 3557-3565.
- Jensen A, Jacobsen JB, Nørgaard M, Yong M, Fryzek JP, et al. (2011) Incidence of bone metastases and skeletal-related events in breast cancer patients: a population-based cohort study in Denmark. BMC Cancer 11(1): 29.
- Coleman RE (1997) Skeletal complications of malignancy. Cancer 80(8): 1588-1594.
- Hofbauer LC, Gori F, Riggs BL, Lacey DL, Dunstan CR, et al. (1999) Stimulation of osteoprotegerin ligand and inhibition of osteoprotegerin production by glucocorticoids in human osteoblastic lineage cells: potential paracrine mechanisms of glucocorticoid-induced osteoporosis 1. Endocrinology 140(10): 4382-4389.
- Rauch A, Seitz S, Baschant U, Schilling AF, Illing A, et al. (2010) Glucocorticoids suppress bone formation by attenuating osteoblast differentiation via the monomeric glucocorticoid receptor. Cell Metab 11(6): 517-531.
© 2018 Ayman Rasmy. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






