- Submissions

Full Text
Novel Approaches in Cancer Study
Parasite Subsistence of Human Cancer
Sergey N Rumyantsev*
Department of Evolutionary Immunology, USA
*Corresponding author:Sergey N Rumyantsev, Department of Evolutionary Immunology, Andent Inc, 31 Cove Lane 6A, 11234 NY, USA
Submission: August 30, 2018;Published: September 17, 2018

ISSN:2637-773XVolume2 Issue2
Abstract
The ability of cancer to subsist in human body like a parasite is out of the mainstream of discoveries of cancer biology, pathogenesis and epidemiology. The goal of this article is to present maximally exhaustive epidemiological, clinical, genetic and immunological evidence of invasive origin and parasite subsistence of cancerous disease. The ability of cancer to invade human body and subsist in it like a parasite is out of the mainstream of discoveries of cancer biology, pathogenesis and epidemiology. The goal of this is to present maximally exhaustive epidemiological, clinical, genetic and immunological evidence of invasive origin and of parasite subsistence of cancerous disease. The investigation is grounded on a multidisciplinary integrative reassessment and reinterpretation of relevant cancer data from the viewpoint of recent achievements in genetic epidemiology, immunology, molecular intra-species ecology and the theory of biological evolution.
Keywords: Cancerous genealogy; Cahexia; Cancerous disease; Cancer surgery; Embryogenesis; Gametes; Ovum; Selfish gene; Sperm; Spermatogenesis; Zygote
Introduction
Cancer is now the biggest threat to global human health
The date of the first cancer appearance is unknown, but its written history starts nearly 4600 years ago from the Egyptian papyrus of around 2625B.C.E., when the Egyptian physician Imhotep described “bulging tumors of the breast”. For therapy, he honestly stated, “There is none” Pederson [1]. One can suppose that cancer is more ancient than the statue of Sphinks. For many subsequent centuries, cancer was not a widely observed disease, killing only some people. It was not until 1940 that cancerous disease overtook many infectious diseases as an important human killer. In the middle of the 20th century, cancer became one of the biggest threats to global human health. Cancer began to take a terrible and growing human toll, and its prevalence continues to grow.
Such dangerous progression cannot happen without a regularly functioning natural mechanism for the transmission of cancer between humans. However, it is not seen as a transmissible disease. The pandemic of cancerous disease has become the quintessential product of modernity. Today, the term “cancerous disease” has more than 100 distinct clinical forms. Each of the forms is named following the organ affected by its initially detected unit. At the same time, all cancerous diseases are thought to share a common pathogenesis Stratton & Rahman [2]. The War on Cancer, the “cancer crusade” forced by the U.S. National Cancer Act of 1971 provided a massive stimulus for cancer research, prevention, and healing. The Act made big promises, promoted the U.S. National Cancer Institute (NCI) and gave the NCI a token measure of independence. Since the 1971 Act, the NCI has spent about $90 billion on science, treatment, and prevention of cancer Marshall [3]. Now, over 40 years later, the disease continues to spread throughout the nation (and the world) with growing intensity. According to Newsweek writer Sharon Begley, “Cancer is on track to kill 565,650 people in the United States this 2113 year - more than 1,500 a day, equivalent to three jumbo jets crashing and killing everyone aboard 365 days a year” Begley [4].
The efficacy of means exploited currently for cancer prevention and treatment appears to be very low. For instance, Provenge, a recent immune treatment for metastatic prostate cancer, costs $93,000 and extends life by about four months Anonymous [5]. Cancer chemotherapy has a 97 percent fatality rate. Really, as Imhotep stated, “There is none” for the therapy of cancerous disease. The over 40 years long war on cancer is proclaimed today as a dismal failure. “Over 40 years We Fought Cancer...And Cancer Won” Begley [4] Why did the Cancer War fail so dismally? The Cancer War failed because the bankruptcy of its theoretical bases.
There exists a lot of cancer features, that united cancer with undoubtedly invasive parasitic diseases. Main of the features are Interethnic differences in susceptibility to cancer; Individual differences in susceptibility to cancer; Individual differences in intrabody locations of cancer affections; Individual differences in intra organ locations of cancer affection the gressivness (gobblenness) the subsistence at the expense of materials, energy and functions of victim The multiplicity of functions that belong to human cancer are performed by a causative agent of the disease, unprecedently specialized monocellular biological entity that evolved to invade human body and to subsist in it at the expense of the materials, energy and functions of the invaded organism. The human cancer has been provided in its evolution by a set of genetic adaptations. Cancerous cells are resistant to defense performed by immune system of invaded organism but ignore physiological regulation of cells dividing and tissues growth. These features are in contradiction to existed paradigm of mutatious origin of initial cancer cell and its subsequent dispersion (metastasis) around invaded human body. What is more, in contrast to other micro-parasites population of cancerous cells exists as a present whole entity. Human populations of West Africa, Abissinia and India are most resistant to cancer.
It was not a war on cancer but the war on cancerous disease. Human cancer is causative agent of human cancerous disease. It is a biological entity adapted, in its evolution, to invade inside of human body and subsist in the genomes of its cells at the expense of stuffs, energy and functions of invaded organism. Over the circle of life, cancer exists sequentially at subcellular, unicellular and multi-cellular forms Rumyantsev [6-8]. It spreads between humans has been intruded inside of the genomes of human reproductive cells either of sperms or ova that function as unicellular host of the parasite. The term cancerous disease united the complex of disturbances induced by the causative agent in the afflicted body.
The NCI elaborated the strategy of the War, but the theoretical base was oriented exclusively 50 years old ago and there has been no revised hypothesis of carcinogenic somatic mutations. The initially accepted paradigm of the origin and pathogenesis of cancer, the ‘somatic mutation hypothesis’ Bauer [9] appeared to be impotent. Most research and treatment questions that vexed the cancer community 40 years ago remain unanswered. The promises of a ‘somatic mutation hypothesis’ appeared unpaid. Rumyantsev [10, 11] A need has emerged to develop a more enlightening paradigm that captures the essentials of the cancer.to perform the first plunge in the insight of cancer existence from the viewpoint of biology.
Poor progress in the knowledge of cancer calls for new research approaches
Compared to the areas in which medical research had its most dramatic successes, cancer presents fundamentally different challenges. New insights in cancer biology, its origin, the circle of life, pathogenesis, clinical progression and epidemic spread are sorely needed Rumyantsev [11,12]. Only one hypothesis can pretend to present a radically different view on the origin, pathogenesis, and pandemic spread of human cancer: the hypothesis of a parasite origin of human cancerous disease Rumyantsev [10,13]. The hypothesis forms the innermost kern of recently elaborated xenogamous (intrusive) paradigm of cancer, which united the issues about cancer’s origins, genetics, mode of existence, pathogenesis, and epidemic spread Rumyantsev [7]. The creation of the entirely different paradigm has been beginning by a set of small pioneer publications offered a new view on the origin of cancer and its pathogenesis, as well as on the mechanisms of its transmission from the diseased persons to the susceptible one. Rumyantsev [6,10,13]. This article aids the most exhaustive search of the hypothesis evidence.
Objective of Research
Objective
To reveal and integrate the first set of biological traits of cancer and to perform the first plunge in the insight of cancer existence from the viewpoint of biology. This approach unites first consecutive analyze of the stages of cancer subsistence beginning from cancerous invasion of a victim body, the potencies of cancer progression within of it prays including the self-procurement of cancer, its impact of on the victim and the sequel of its circle of life by the transmission between humans.
Justification of research
The need of this research is justified by the impotency of current oncology to prevent and heal cancerous disease which is now the biggest threat to global human health. The failure arose because current medicine lucks appropriate knowledge of cancer origin, the manner of its existence and dispersion among humans. For many years these basic questions were out of the main stream of oncology, epidemiology and medicine. The results of discussed research will be able to form the milestone in this scientific domain.
Experimental
The main problem to be solved is to reveal and integrate the set of cancer traits characterizing the biology of any cancerous entity. The set should include the cancer invasion, its initial development and dispersion inside of invaded human, the foreignness of cancerous tumors for its host, the ability of cancer to withstand self-defense of its pray, the self-procurement of cancer life, impact of cancer on its victim as well as the self-reproduction of Cancer and its transmission between humans. The investigation is based on a multidisciplinary approach to integrative reassessment and reinterpretation of relevant current data about cancer epidemiology, molecular pathogenesis, clinical manifestations and the recent achievements of up-to-date, all-pathological, immunogenetic, genetic, and evolutionary discoveries performed at cellular, subcellular and molecular levels. The focus is on the consecutive stages of cancer subsistence, beginning from the initial invasion of victim’s genome by a cancerous gamete up to the transmission of cancerous genome between people. At every level of the search, the special attention was focused on identification of the tremendous diversity of relevant traits, especially of those involved in the formation of the phenomenon of hereditary immune mosaicism. Rumyantsev [14,15]. Various appropriate data from the literature has been summarized with the data of long-term investigations performed by the author and the team he leads. Rumyantsev [16].
Result and Discussion
The stages of cancer subsistence
Human cancer, causative agent of human cancerous disease is a biological entity adapted, in its evolution, to invade inside of human body and subsist in the genomes of its cells at the expense of stuffs, energy and functions of invaded organism. Over the circle of life, cancer exists sequentially at subcellular, unicellular and multi-cellular forms. It spreads between humans has been intruded inside of the genomes of human reproductive cells either of sperms or ova that function as unicellular host of the parasite. The core of cancer consists of a set of genes, controlling the development of ripe stages of the parasite as well as its physiological and ecological functions. This set performs the functions of cancer genome. It consists of genes foreign to it pray and exists in the pray’s genome like a xenogamous intruder. Its structure can control the performance of traits intrinsic to any other parasite including nutrition and self-reproduction. Besides, cancer possess many original adaptations to parasite way of life.
Cancer circle of life
The consequent events of cancer life can be present in the form of its specific Circle of Life (Figure 1). This circle can be performed by cancer only inside of the human body at the expense of relevant stuffs, energy and functions of invaded organism. The assort cement of host prerequisites for the development of cancer varied over the progression of cancer.
figure 1:Integrative analytical model of cancer circle of life.
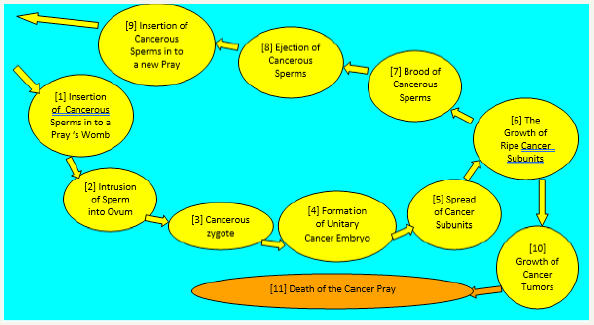
Cancer invasion of a victim’s body
Cancer is initiated by the appearance in the human body of deviant cell lineages that habitual regulators of cell dividing, and tissue growth are unable to control. The uncontrollability is predetermined by the constitutional immunity of cancerous cells to the mediators of habitual regulation of cell dividing and tissue growth Rumyantsev [10,13]. This intrinsic trait of cancerous cells is their ultimate evolutionary adaptation for carcinogenesis. Such deviant cell lineages appear in the human body as a result of genome transformation performed over the heterozygous crossbreeding between parental gametes with partially different (divergent) genotypes. Over xenogamous formation of a descendant’s zygote, its genome becomes admixed with carcinogenic genes Rumyantsev [12].
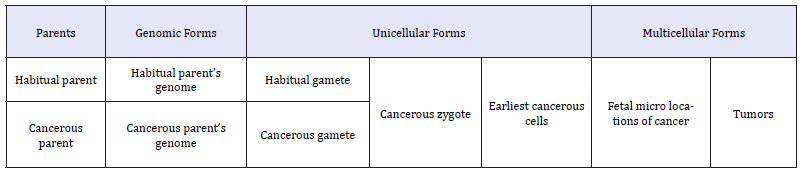
Xenogamous mating between members of genetically different species, subspecies, ethnoses and families led to the intrusion of a genome with components of deviant genetic information that induced intra-individual diversity of cell lineages Rumyantsev [17]. Some of the cells appear to own the main trait of cancerous cells, genetic immunity to habitual regulators of cell division. The descent and consequent subsistence of human cancer includes regular obligatory alternation of successive descendants, which formed a hypothetical pathway of tumor development from gametes-zygote to an advanced-stage of cancer (Table 1).
Table 1:Successive forms in cancer progression and subsistence (according to Rumyantsev [8] updated).
The coexistence in a xenogamous zygote of both habitual and deviant, for instance, cancerous genes is a result of well-known of mechanism of heterozygous interbreeding, which is responsible for the formation of the intra-individual biodiversity characteristic of any kind of human pathology. Rumyantsev [7,14] and Gerasimov [18]. After the development of the zygous form, the descendant organism consists of both habitual and deviant cells. The activity of cancerous genes leads to the appearance in the invaded human body of a set of deviant cell lineages provided with relevant cancerous abilities. They are able to resist the habitual regulation of cell division and tissue growth as well as withstand the victim’s immune response. The lineages and their extracellular associates first form the micro-locations of cancer units and then their clinically detectable locations, the cancerous tumors.
It should be especially accentuated exceptional foreignness of cancer for its host. All cancer looks alien in the body afflicted by them. This is applicable both to the bodies of cancerous tumors (Figure 2) and their microscopic cellular and tissue structures. There exist a plenty of various morphological and physiological manifestations of the foreignness of cancer for its prey. Some of them may remind the similar but not identical traits of those ones if any of infectious and parasite diseases. Their influence is revealed in any other features of cancer both unique and universal all-pathological traits of malignancy as well. The presences of cancerous foreignness were surely evidenced in lung and breast cancers. (Over 90%) by dogs’ scent Mc Culloch et al. [19].
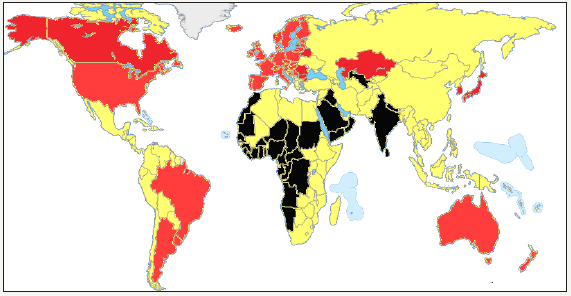
figure 2:Geographic dispositions of current human populations differing in susceptibility to cancer after the Grate Geographic Discoveries Rumyantsev 2016.
Embryogenesis of cancer
The early postzygotic stages of human embryogenesis are not enough for current discovery. It is necessary only to accentuate that divergence between normal and aberrant cell clones could begin far before antenatal embryogenesis. Earliest cancerous cells are formed fetal micro-locations dispersed around the body in accordance with general rules of embryonic differentiation of tissues and their dislocations inside appropriate organs. Rumyantsev [8].
Analogous phenomenon of mosaic disposition has a brilliant track record in the fields of infectious diseases. Like the clones susceptible to infectious agents, any aberrant cell clones are usually present among the clones of habitual cells but in a far lesser quantity. Rumyantsev & Gerasimov [18] and Rumyantsev [20]. In one case of sickle cell anemia, aberrant erythrocytes consisted of 22 percent of the total number of red blood cells. Analogous phenomenon of dispersion mosaicism has a brilliant track record in the fields of infectious diseases. Individual variations in the sizes and focal locations of relevant susceptible cell clones can be seen also during the observation of many infectious diseases.
Dispersion of observed clones can be extremely variable in the number and size of locations. The number of patches may be less than a dozen in a minor illness, or they may number in the thousands in a more severe case of the same kind of disease. Beyond the edge of aberrant location, the regular tissue is normal. All the discussed traits of the dispersion of cell clones susceptible to relevant infectious agents (places of locations, their number and sizes) are formed before postnatal ontogenesis. Rumyantsev [16] and Gerasimov [18]. This may mean that distribution of aberrant clones is programmed by genomes. Cancerous cells also appear in and stochastically disperse around the victim’s body before postnatal ontogenesis and initially exist in it as subpopulations (units) of smaller but different sizes. For instance, prostate cancer is a form of malignancy which mainly develops in the prostate. Its additional units become visible later and are mainly located in the bones and lymph nodes. Prostate cancer tends to develop in men over the age of 50 Siegel [21]. The genomic roots of these traits should be subject to special investigation. In contrast to their steadfast locations, cancerous units enlarge during their postnatal life. The primordial and late appearing subpopulations of cancerous cells and the tu mors formed by them far later reside stably in their initial places in different areas of the body. They do not metastasize. In reality we can only observe non-simultaneous appearance of several identical tumors in different parts of a diseased body. This explanation of the reasons and propelling forces of cancer’s discretion has been proposed and developed only recently Rumyantsev [10,11,13].
Dispersion of cancerous units around the victim’s body
According to well established knowledge, Gilbert [22] every new entity is initiated by the process of fertilization involving the fusion of male and female gametes to form a zygote, the unicellular form of the entity born during the fusion of gametes and their genomes. In the case of carcinogenic fertilization, the zygote’s genome will contain carcinogenic components. Immediately following fertilization, the zygote undergoes a series of extremely rapid mitotic divisions (cleavages) wherein the enormous volume of its cytoplasm is divided into numerous smaller cells (blastomeres). In the case of the carcinogenic zygote, some of the blastomeres may contain cancerous components in their genomes.
By the end of cleavage, the blastomeres form an unfilled spheroid known as a blastula and then change their positions relative to one another. This series of extensive cell rearrangements leads to the formation within the embryonic entity of three germ layers: the ectoderm, the endoderm, and the mesoderm. The layers interact with one another and rearrange themselves to produce tissues and organs. The developing entity enters the stage of organogenesis. During organogenesis, certain cells undergo long migrations from their places of origin to their final locations. These migrating cells include the precursors of blood cells, lymph cells, pigment cells, and gametes. Many organs are formed of cells from more than one germ layer. For instance, most facial bones are derived from cells that have migrated ventrally from the dorsal region of the head. A specialized portion of zygote cytoplasm gives rise to cells that are the precursors of the gametes (the sperm and egg).
The gametes and their precursor cells are set aside for the function of reproduction. The separation of somatic cells (which give rise to the individual body) and germ cells (which contribute to the formation of a new generation) is often one of the first differentiations to occur during animal development. The germ cells eventually migrate to the gonads, where they differentiate into gametes. The development of gametes is usually not completed until the organism has become physically mature. Gametogenesis begins during development but is completed in the sexually mature adult. At maturity, the gametes may be released and participate in fertilization to begin a new embryo.
At least two paradoxes can be seen in the disposition of either primordial or later appeared malignant tumors. Firstly, in contrast to assumed ubiquitousness of primordial tumors there are both more favorite and far fewer favorite sites of their dispositions. The primordial tumors are mainly disposed at prostate, lung, bronchus, colon, urinary bladder, skin, kidney, rectum. pancreas, stomach. Besides, hypopharynx, bones and joints, floor of mouth, nasopharynx, gallbladder, oropharynx, oral cavity, trachea, peritoneum and pleura are far less favorable for the disposition of primary tumors. Secondly, there are only some most common sites where the late appeared tumors are preferably disposing - the lungs, bones, liver, and brain. Other places of a body are seen far less accessible for appeared tumors. One question arises immediately - are these unfavorable places immune to the invasion of cancer? The way of existing of such variation as well as its reasons have not been discussed anywhere before.
Over stages of organogenesis, the earliest primordial cancerous cells are carried to different areas of the embryo’s body before postnatal ontogeny in the same manner that is used to create other embryonic tissues and organs. After the end of their dispersion and initial multiplication, the cells exist like the primordia of future tumors, sleeping cell masses of smaller but different sizes. The carcinogenic components in the cell’s genomes may dispose at various places of the afflicted entity, probably according to their intrinsic predilection. After that the cells continue to exist inside the infected body in the form of several distantly separated micro-populations, the cancerous subunits, and provided with life- supporting stuffs and energy by the organism. The development of detectable tumor is usually delayed for decades. At the appropriate time of the host’s life (mainly after 40 years of age), probably according to a specific program of cancer ontogenesis and aging, the potentially cancerous micro-populations receive their specific impulse to awaken. This means that human cancer possesses its own schedule, an intrinsic biological watch; i.e., the genetic program of its development from zygote and primordial cancerous cells to transmission between humans. This programmed cancer subsistence is different of that of its victim. This is a specific cancerous germ line - the lineage of cells culminating in the germ cells. The possession of these unique genomic traits provided cancer with many benefits of undoubtedly adaptive importance. The program favors those cancerous cell lineages whose schedule of life does not allow early restriction of reproductive, i.e transmissive, functions of the afflicted person as well as the period of its effective care for offspring before its victim is 40 years of age.
Heterogenic make-up of a ripe cancer
The somatic mutation hypothesis allows the existence of only one cancerous cell clone in an affected body. First doubts about this hypothesis were revealed by integrative analyses of epidemiological and clinical observations, Rumyantsev [16] according to which the multiple cancers comprise two or more primary cancers occurring in an individual that originate from a primary site or tissue and are neither an extension nor a recurrence or metastasis Soerjomataram & Coebergh [23].
Cancer patients have a 20% higher risk of a new primary cancer compared to the general population. Approximately one third of cancer survivors aged >60 years were diagnosed more than once with another cancer. As the number of cancer survivors and older people increases, the occurrence of multiple primary cancers is also likely to increase. Levi et al. [24], Milan et al. [25], Nugent et al. [26], Soerjomataram et al. [27] and Soerjomataram & Coebergh [23].
Such observations prompted the idea of the possible existence of several appropriate clones in cancerous tissue. This means that like any other multicellular being, cancer may contain a variety of different cells and associated extracellular structures that are under different genetic regulation and may perform different functions at different stages of cancer development. Rumyantsev [10,11]. According to the xenogamous theory of carcinogenesis, any ripe cancer should consist of several subunits of various sizes that are positioned in different areas of the afflicted body. Each subunit contains cellular and tissue structures. In contrast to the somatic mutation hypothesis, the existence in a cancer of several different clones was recently documented very well.
Recent studies, Kreso et al. [28] together with the set of data discussed above, allow us to suppose that like any other multicellular being, cancer contains a variety of different cells that are under different genetic regulation and possess different behaviors. Cancer consists of a couple of functionally heterogeneous cell lineages that vary with respect to their distinctive structural or physiological functions and potentials. The heterogeneity within a tumor cell lineage may also determine the differences within the tumors and their locations. Cancer is able to maintain its structural stability through many generations and the diversity of cancer composition remains stable over its sequential long-term propagation Kreso et al. [28]. This means that both animal and human cancers have developed many adaptations that enable these aberrant lineages of mammalian cells to exist as a multicellular parasite Rumyantsev [10,11].
Cancer cells are the driving force of tumor development and progression, yet these transformed cells cannot do it alone. Assemblages of ostensibly normal tissue and bone marrow-derived (stromal) cells are recruited to constitute tumorigenic microenvironments. Most of the hallmarks of cancer are enabled and sustained to varying degrees through contributions from repertoires of stromal cell types and distinctive sub-cell types. Their contributory functions are becoming increasingly better understood, as are their reciprocal communications with neoplastic cancer cells that mediate their recruitment, activation, programming, and persistence Hanahan & Coussens [29].
Such complicated traits cannot belong to a lone cell. Besides, their acquisition cannot be achieved by single mutation. This conclusion discredits the basis of the somatic mutation hypothesis but supports the compromising idea of cancer occurring as a consecutive accumulation of mutation upon mutation on a single normal cell. Hanahan & Coussens [29] and Hanahan & Weinberg [30]. The new versions of the somatic mutation hypothesis do not discuss cancer transmission between humans either. Cancerous tumors are composed of multiple cell types: stromal, immune or malignant cells. Malignant cells can also show sub-clonal heterogeneity, where different clones carry various somatic mutations and show variable oncogenic potential or drug sensitivity. Finally, this sub-clonal population can change during the progression of the cancer Mardis [31]. Cancer is sustained by the production of aberrant cells that vary in many morphological and physiological properties. The repopulation dynamics of 150 single lineages from ten human colorectal cancers were followed. The revealed functional heterogeneity of the cell lineages varied with respect to their distinctive structural or physiological functions and potentials. Some clones were able to become dormant and undetectable only to become abundant in later generations Kreso et al. [28].
Heterogeneity within a couple of tumor cell lineages may also determine the differences within the kinds of tumors and their locations. Cancer maintains its heterogeneous structural stability through many generations. The diversity of cancer composition remains stable over its sequential long-term propagation. Kreso et al. [28]. The presence of various slow-growing dormant clones was also evidenced by the re-emergence of previously minor clones after chemotherapy, and their ability to initiate new tumors (although of a smaller size) over subsequent transplantations of the tumors in experiments Marusyk & Polyak [32].
Incipient micro-populations of cancerous cells are formed, distributed and dispersed in the afflicted body before postnatal ontogenesis in the form of distantly separated micro- populations and their initial sizes are different but very small. The cancerous subunits are dispersed around the body either stochastically or in a manner not yet understood. Accordingly, the formation of subunits before postnatal ontogenesis is the reason they are not eliminated by the mechanisms of adaptive immunity performed by the lymphatic system Rumyantsev [11].
It was supposed that cancerous units in primordially different locations become clinically detectable at different times after initiation of malignant growth; this allowed for the differences in their initially smaller sizes. The differences in initial cancer cell masses and their subunits around the body predestine individual diversity in the course and severity of cancer when the disease begins to develop Rumyantsev [12]. At a relevant time in a victim’s life, the uncontrollable growth of such micro-subpopulations becomes visible in the form of detectable extra cell masses of cancerous tissue, the malignant tumors. The largest of the primordial subpopulations achieves the size of detectable tumor far earlier than the smaller ones, thus forming the first apparent cell mass, usually called the ‘primary’ tumor. The subpopulations of initially lesser sizes may become visible in the form of ‘secondary’ detectable tumors.
Hereditary immunity of cancer to victim regulatory management
Any living being is constitutionally provided with a physiological system that maintains normal body structure within its genetically predetermined shape, size and function. A special part of this very important and effective system is dedicated to managing the starting and revival of body structures and functions on molecular, sub cellular, cellular, tissue and organ levels. Habitual cells of normal organisms grow and divide to form new cells as the body needs them. When cells grow old and die, new cells take their place. The regulation is realized on the cellular level and performed by means of molecular humoral agents.
In the case of cancer invasion, this orderly process goes wrong. The mighty system of body management and maintenance appears to be impotent, even in relation to some its initially smallest parts, the subunits of cancer. Cancerous cells grow and divide independently of habitual physiological management. That occurs because cancer cells and tissues possess absolute constitutional immunity to the agents of habitual physiological management of cell division and tissue formation. Constitutional (hereditary) immunity of the cells against relevant physiological regulators can be created by structural incongruence between regulators and their receptors. The existence of such specific immunity is considered the obligatory prerequisite to malignity Rumyantsev [11].
Cancer cells continue dividing and forming the masses of relevant tissue when the afflicted body does not need them. Furthermore, the cancerous cells of older generations do not die when their peers would. The extra cells form the masses of tissue called malignant tumors. This innate (constitutional) trait of cancerous cells is of most adaptive, pathogenic importance. This innate immunity of cancerous cells functions in all stages of cancer and maintains its initiation, development and subsequent progression.
Hereditary immunity of cancer to victim immune defense
Human cancer invades its victim because there is no immunity. The malignant cells and tissues are inherently protected from destruction by cell and humoral mechanisms launched by the victim’s lymphatic system of responsive immunogenesis. Cancerous cells are not recognized by the victim’s immune system as non-self because their surface does not contain relevant molecules of the major histocompatibility complex that are essential to the antigen- processing pathway. Such traits allow the cancer to evade the surveillance performed by the victim’s system of immunogenesis. This protection is predetermined by the germ line of the formation of cancerous cells directly from the zygote over the prenatal development of the afflicted organism Rumyantsev [10]. This trait of cancer ontogeny is undoubtedly of evolutionary adaptation, providing the parasite with a lifelong ability to escape rejection by the victim’s immune response.
The self-procurement of cancer
Like most other living beings, the cancerous entity is the heterotroph that sustains itself at the expense of substances and energy derived from its environments, i.e., from the body of its prey. Any individual cancer exists as a case of natural ecological relations between two living species in which the consumer obtains the energy for its life at the expense of substances and physiological functions composed of the consumed (the victim). What is more, the populations of cancerous cells subsist also on life-supporting functions (nutrition, respiration, circulation of blood and lymph) belonging to the victim. Thus, like in any other internal parasite, cancer is a kind of ultimate parasite. Many evolutionary adaptations of cancer are due to its managing its own nutrition.
The extraction of nutrients from a victim’s body by cancerous cells is extraordinarily intense and thus is a leading cause of poor quality of life, poor physical function, and poor prognosis in cancer patients DeWys et al. [33]. Cancer’s self-provision nutrients cause damage to the victim, its reduced vitality and finally death. Human cancer is a highly exceptional man-eater. The ultimate state of cancer is characterized by cancerous cachexia, a catastrophically progressive weight loss provoked by intensive atrophy, mainly of skeletal muscle and adipose tissue, which are used as the main sources of lipids and proteins. Depending on the tumor type, weight loss occurs in 30-80% of cancer patients and is severe (with loss of >10% of the initial body weight) in 15% of cases. DeWys et al. [33] In pancreatic cancer, 85% of patients become cachectic even at diagnosis Tisdale [34].
The Progression of Cancer Within of the Victim’s Body
Impact of cancer on the victim
The complex interactions among molecules by which cancer can influence its victim and affect his or her structures and functions are only now beginning to be elucidated. Solid cancers cannot grow beyond a certain size without an adequate blood supply Carmeliet & Rakesh [35]. The hypothesis that tumors produce a diffusible ‘angiogenic’ substance was put forward in 1968 Ehrmann et al. [36]. Cancer units produce humoral factors that are able both to induce and promote angiogenesis; Hanin [37] this is addressed toward each of them individually and thus perform an “angiogenic switch” of their own unrestricted growth.
Angiogenesis is a critical, rate-limiting step in the multi-stage process leading to a detectable cancerous unit. The induction of angiogenesis is an important step in carcinogenesis. This angiogenic activity first appears in a subset of hyperplastic islets before the onset of tumor growth Folkman et al. [38]. An angiogenic switch causes the tumor to advance down the progression pipeline Hanin [37]. One can hypothesize that it is a specific cancerous vascular endothelial growth factor, a signal protein produced by cells that stimulates vasculogenesis and angiogenesis and restores the oxygen and nutrient supply to cancerous units when the local blood circulation is inadequate.
The cancerous atrophy of skeletal muscle is characterized by an intense degradation of the macromolecules of muscle proteins and the depression of their biosynthesis. The associated massive loss of adipose tissue is incited by extensive degradation of fat molecules. Cancer functions as a marauder which sucks up the body of its victim until it is about dry. In addition, one can suppose some cyto-ecological regulators produced by cancerous cells inhibit the growth of normal cells, thus aggravating the cancerous cachexia. Some of humoral agents of cancerous cells suppress the functions of the victim’s cells, thus contributing to the development of cachexia Tisdale [34].
The development of this state is induced by the primordial existence in the afflicted organism of a symbiotic population of cancerous cells. The population exists inside the afflicted organism like a sponge. It develops intensively at the expense of both the structures (proteins, lipids, saccharides) and functions (the supply of oxygen, nutritive substances and means for reproduction) of the host’s organism. The cells are able to produce molecular agents specifically targeted on the enzymatic splitting of muscle proteins. Besides, cancerous cells can secrete lipolytic enzymes which functions make substantial investment in the creation of cancerous cachexia.
When a victim dies of cancer, it is mostly because its tumors have exhausted its life-supporting processes and intoxicated its life-supporting organs. Cancer gobbles up its host. The development of either solitary or associated malignant tumors inevitably leads to the death of the host long before the genetically predetermined limit of its longevity. This marauding way of life exploited by cancerous tumors (the populations of cancerous cells and their subcellular structures) is performed mainly by molecular enzymatic agents, targeting either the splitting of the host’s macromolecules or producing a functional inhibition of their cells. The possession by cancer of such specialized and undoubtedly adaptive toxins and nutritive factors is evidence of the evolutionary origin of cancer’s marauding nature.
The set of spatially separated cancer subunits functions like the integral whole, the united organism consisting of many homologous sub organisms, a kind of multicellular organism. This undoubtedly adaptive trait enhances the ability of the invading parasite to colonize in the maximal quantity of locations in the victim’s body that is appropriate for further development.
The earliest primordial cancerous cells settle in various areas of the embryo’s body before postnatal ontogeny in the same manner that is used to create other embryonic tissues and organs. After the end of their dispersion and initial multiplication, the cells exist in their places like the primordia of future tumors, small sleeping cell masses of varying size. The cells continue to exist within the infected body in the form of several distantly separated micro-populations, being provided with life support and energy by the organism. The further progression of cancer may be delayed for decades.
At the appropriate time of the host’s life, the potentially cancerous micro-populations receive their specific impulse to awaken. This means that human cancer possesses its own schedule, an intrinsic biological watch; i.e., the genetic program of its development from zygote and primordial cancerous cells to transmission between humans. This programmed cancer subsistence is different of that of its victim. This is a specific cancerous germ line - the lineage of cells culminating in the germ cells. The possession of these unique genomic traits provided cancer with many benefits of undoubtedly adaptive importance. The program favors those cancerous cell lineages whose schedule of life does not allow early restriction of reproductive, or transmissive, functions of the afflicted person as well as the period of its effective care for offspring.
At the appropriate time of the host’s life, the potentially cancerous micro-populations receive their specific impulse to awaken. This means that human cancer possesses its own schedule, an intrinsic biological watch; i.e., the genetic program of its development from zygote and primordial cancerous cells to transmission between humans. This programmed cancer subsistence is different of that of its victim. This is a specific cancerous germ line - the lineage of cells culminating in the germ cells. The possession of these unique genomic traits provided cancer with many benefits of undoubtedly adaptive importance. The program favors those cancerous cell lineages whose schedule of life does not allow early restriction of reproductive, or transmissive, functions of the afflicted person as well as the period of its effective care for offspring.
At the appropriate time of the host’s life, the potentially cancerous micro-populations receive their specific impulse to awaken. This means that human cancer possesses its own schedule, an intrinsic biological watch; i.e., the genetic program of its development from zygote and primordial cancerous cells to transmission between humans. This programmed cancer subsistence is different of that of its victim. This is a specific cancerous germ line - the lineage of cells culminating in the germ cells. The possession of these unique genomic traits provided cancer with many benefits of undoubtedly adaptive importance. The program favors those cancerous cell lineages whose schedule of life does not allow early restriction of reproductive, or transmissive, functions of the afflicted person as well as the period of its effective care for offspring.
Cancerous cachexia
Cancerous cachexia is the ultimate state of the cancer disease and is characterized by a catastrophically progressive weight loss provoked by intensive atrophy of, mainly, skeletal muscle and adipose tissue. The cancerous atrophy of skeletal muscle is characterized by an intense degradation of muscle protein associated with the depression of protein biosynthesis. The massive loss of adipose tissue is incited by extensive fat degradation. Cancer functions here as a marauder which sucks up the body of its victims until it is just about dry. Furthermore, one can suppose some cyto-ecological regulators produced by cancerous cells inhibit the growth of normal cells, thus aggravating cancerous cachexy.
The development of this state is induced by the primordial existence of a population of xenogeneic symbiotic cells that exist inside the afflicted organism like a sponge. It develops intensively at the expense of both the structures (proteins, lipids, saccharides) and functions (supply of oxygen, nutritive substances and means for reproduction) of the host’s organism. The cells can produce molecular agents specifically targeted at the enzymatic splitting of muscle proteins. Furthermore, cancerous cells secrete lipolytic enzymes, which make a substantial contribution to the creation of cancerous cachexia.
When a host dies from cancer, it is mostly because the tumors have exhausted its life- supporting processes and intoxicated its life-supporting organs. Unfortunately, the discovery of the molecular origin of cancerous intoxication is only recent. The development of either solitary or associated malignant tumors inevitably leads to the death of the cancer’s host long before the genetically predetermined limit of its longevity.
Self-regulation of cancer
Usually cancer consists of some separate units that are dispersed around the victim’s body. However, they keep their physiological unity. This is demonstrated by the set of unique traits recently evidenced in post- surgical and experimental observations.
Communications between cancerous units
The existence of inter-tumor communications was hypothesized in Prehn [39] and confirmed in a host of other studies, many of which are reviewed in Retsky et al. [40] and Rumyantsev [7]. It was noted that large tumors inhibit the growth of smaller tumors and thwart the inception of new tumors. Baum et al. [41], Demicheli et al. [42], Hanin & Korosteleva [43] and Retsky et al. [40]. Extirpation of larger tumors triggers the accelerated proliferation of smaller, dormant or slower-growing cancerous units. The removal of the primary tumor could accelerate the growth of subunits which were inhibited before. The accelerated progression of cancerous units after foregoing resection was noted in experimental De Jong et al. [44], Garcia-Alonso et al. [45], Ikeda et al. [46] and clinical Elias et al. [47] and von Schweinitz et al. [48] studies.
Acceleration in the rate of growth of secondary subunits was found after 70% ectomy of cancerous liver Sorin et al. [49]. Resection of other primary tumors was followed by a 32-fold increase in the rate of the growth of secondary tumors Hanin & Korosteleva [43]. More importantly, the early extirpation of the first apparent cancer unit does not prevent the subsequent appearance of “secondary” units. Giuliano et al. [50] and Pockaj et al. [51]. This may mean that at the time of the resection, the secondary tumors already existed in the form of undetectable micro-populations.
It is proposed that tumors produce humoral factors able either to promote or inhibit tumor growth and angiogenesis. Removal of the primary tumor reduces the production of growth inhibitors and pro-apoptosis factors and signals, which accelerates the growth of smaller subunits Hanin & Korosteleva [43]. This important finding has been directly confirmed in several well-documented clinical case studies involving various types of cancer. For instance, in eight cases of testicular cancer, resection of voluminous tumors caused a dramatic exacerbation of the disease Lange et al. [52]. Excision of primary melanomas precipitated the appearance of new subunits in three skin cancer patients. De Giorgi et al. [53], Tseng et al. [54]. In one case of pancreatic cancer, excision of the primary adenocarcinoma caused the appearance in the liver of numerous previously undetectable subunits Deylgat et al. [55].
A woman diagnosed with breast cancer had a tumor of 10.3cm3 in volume. The tumor was resected. However, eight years after resection, 37 previously undetectable cancerous units were discovered in her bones, lung, lymph nodes and soft tissue. The volume of 31 bone tumors varied from 1.69 to 22.96cm3. Three lung tumors varied from 1.30 to 7.26cm3, two lymph node tumors were 2.85 and 9.66cm3, and one tumor was 11.41cm3. In two other breast cancer patients, 20 and 15 bone tumors became detectable five and a half years and nine months after primary resection, respectively Hanin & Korosteleva [43]. Thus, the life of all subpopulations of cancerous cells is controlled by their own united physiological mechanism which maintains the whole structure of cancer within a genetically predetermined size. The destruction of one or more subunits boosts the growth of the others. The set of separated subunits functions like the integral whole, a physiologically and ecologically united organism consisting of many identical sub-organisms. This is a kind of multicellular superorganism.
Physiological synchronization between cancerous units
Human cancer possesses have their own schedule (the program of ontogenesis) as well as the ability of physiological synchronization between its distant separated units. The existence of these intrinsic traits has been presented and discussed in detail. Rumyantsev [11], Rumyantsev [10], Rumyantsev [7] and Rumyantsev [13]. It was proposed that cancer genomes contain a functional program of development, alternating its successive forms over time. The existence of these traits has been supported both by clinical observations and experiments Rumyantsev [10-13].
Self-reproduction of cancer
The saving and continuing of its own life via self-reproduction and consequent transposition from the location of exploited resources toward unexploited ones is an extraordinarily important function of every form of living matter. Human cancer also performs these functions very regularly and effectively by means of human reproductive organs and functions. This peculiar form of life is characterized by a complex of evolutionary adaptive traits necessary for the transmission of deviant genomes into relevant gametes, the execution of multifold acts of copulation, fertilization, giving birth and breeding descendants to the stage of complete maturity. The absence of any of these abilities sharply diminishes the chances of the cancerous genome prolonging its life in the genomes of descendant generations.
This set of relevant functions is performed through exploitation of the host’s reproductive system. Undoubtedly, natural selection favors those cancerous cell lineages whose genomic schedule of life did not restrict their productive, i.e. transmissible, function of the afflicted person nor its care for its offspring up to the maturation of reproductive (transmissible) stage. What is more, there is increasing evidence that some forms of cancer may be able to stimulate the reproductive functions of their hosts.
The risk of cervical cancer is influenced by factors related to a woman’s sexual activity history, specifically, her age at her first sexual encounter and the number of her sexual partners Skegg et al. [56]. Furthermore, significant difference between patients and control subjects was obtained for the development of cervical cancer among wives when husbands had three or more extramarital sexual partners. Cervical cancer occurs most often in women who had multiple sexual partners, who also had multiple sexual partners. It is mainly a disease of prostitutes and promiscuous women. Among sexually monogamous women, male sexual partners play a significant role in cervical carcinogenesis. When husbands had sexual relationships both before and during the marriage, their wives’ risk of getting cervical cancer increased. Male promiscuity plays a significant role in the etiopathogenesis of cervical cancer. History of sexually-transmitted disease before marriage or after marriage is an important risk factor Shyman et al. [57].
Prostate cancer is also associated with promiscuity. A new study has found that promiscuity in a man’s younger years could increase his risk of developing prostate cancer when he gets older. The risk of prostate cancer increases directly with the lifetime number of female sexual partners but not with male partners. Rosenblatt et al. [58]. Men reporting 25 or more sexual partners were 2.80 times more likely to be diagnosed with cancer compared to men with five or fewer partners Sarma et al. [59].
Some small size genomic ingredients, the selfish genes, are known mostly benign commensal components which invade the genomes of sexual populations despite conferring no benefit to their eukaryote hosts. However, some selfish genes may be genomic parasite. They demonstrate both reproduction and transmission bias and thus confer benefits for its own existence Werren [60]. What is more, according to recent observations some selfish genes may increase the propensity of its eukaryote host to undergo sex with increased promiscuity and along with increased rates of non-Mendelian inheritance, this may promote spread Giraldo-Perez & Goddard [61]. These observations along with above discovered association of promiscuity with ethiopathogenesis of some cancer allow us to propose that appropriate cancerous gametes contain ingredient analogous to above discussed selfish genes.
The transmission of cancer
The intrusion of infectious agents inside the next victim’s body is mainly carried out by means of the victim’s ecological communications, through which the regular physiological functions are provided; for example, through feeding (as an alimentary intrusion), breathing (respiratory intrusion), as well as through direct contact and self-reproduction (venereal intrusion). Of the three, developed the alimentary transfer of infectious agents functions the most widely and effectively Burgasov & Rumyantsev [62]. Sexual intrusion is also quite intensive.
Before the paradigm of the parasitic nature of human cancer was developed, Rumyantsev [7,10,11,13] there were no proposed naturally occurring ways for the transmission and spread of cancer between humans. The prevailing hypothesis of a stochastic origin of any cancer out of somatic mutation of a single cell did not allow for even the thought of cancer transmission between people.
There were only rare reports of artificial cancer transmission between humans by an accidental transfer of cancer cells through organ transplantation or during surgical procedures as well as the problematic transfer of cancer cells from mother or co-twin via the placenta. Only 0.04% of organ transplant recipients contracted cancer from the donor organ. Furthermore, the survival of transplanted cancers in healthy humans is exceedingly rare and documented in only a handful of cases. Genetic immunity probably prevented such cancers from taking hold Rumyantsev [7]. Meanwhile, human cancer is also characterized by the set of some trait’s characteristic of malignant growth naturally transmissible among animals. Relevant information about the totality of these traits has recently been summarized and interpreted Rumyantsev [7].
Undoubted analogies can be seen in the prevalence, clinical exposure and progression of disease, the origin of causative agents, and especially in the genetic deviation’s characteristic of both animal and human malignancies. Any cancer sustains itself at the expense of substances in the host’s body. This set of traits includes the abnormal reproduction of some aberrant cells and consequent growth of relevant aberrant tissues in different parts of the afflicted organism. Both animal and human cancers are able to exhaust the life-supporting functions of the invaded body and intoxicate its lifesupporting organs.
Recent studies, Kreso et al. [28] together with the set of data discussed above, allow us to suppose that like any other multicellular being, cancer contains a variety of different cells that are under different genetic regulation and possess different behaviors. Cancer consists of a couple of functionally heterogeneous cell lineages that vary with respect to their distinctive structural or physiological functions and potentials. The heterogeneity within tumor cell lineages may also determine the differences within the kinds of tumors and their locations. Cancer can maintain its structural stability through many generations and the diversity of cancer composition remains stable over its sequential long-term propagation Kreso et al. [28]. These mean that both animal and human cancers have developed many adaptations that enable these aberrant lineages of mammalian cells to exist as a multicellular parasite Rumyantsev [10,11].
Sexual transfer of cancer via reproduction of its prey
According to the set of evidence discussed above, human cancers belong to the group of deadly invasive diseases. Infections and parasitic invasions belong to this group too. Like any other deadly invasive disease, human cancer exists because of natural ecological relations between two species, in which the contagious species (the consumer) obtains the matter and energy for its life, with reproduction and subsequent transmission at the expense of substances contained in the living victim. These actions exhaust the lifeblood of the afflicted body and thus restrict its vitality, provoking the state of disease and a loss of victim viability. Prey is not available for the subsistence of every cancer. Cancer must live within the exhausted victim before it dies. Consequently, the subsistence of such diseases depends on regular transmission of the causative agent from one living victim to the next one.
The transmission of any invasive parasite inside the body of the next victim is mainly carried out by means of the victim’s ecological communications, through which the regular physiological functions of nutrition, respiration, and self-reproduction are provided (alimentary, respiratory, and sexual transmission). Transmissible canine venereal cancer is a parasitic disease of dogs and other canines. It passes from one dog to another, usually during coitus. Its invasive agents are the tumor cells themselves Murgia et al. [63]. Some studies have estimated that the disease perhaps originated from a wolf or an East Asian breed of dog between 200 and 2500 years ago Murgia et al. [63] or more than 6000 years ago when dogs were first domesticated. Rebbeck et al. [64]. According to a more recent estimation, the lineage of malignant cells first arose in a dog with low genomic heterozygosity that may have lived about 11,000 years ago. The cancer spawned by this founder dispersed across continents about 500 years ago in the era of rapid human global exploration.
The genomes of cells that form the canine venereal tumor contain 1.9million somatic substitutions in contrast to the genomes of the organisms consumed by them Murchison et al. [65]. The genomes of tumors from different cases show very little microsatellite variation. The invasive agents have gone through many remarkable adaptations that have enabled this mammalian cell lineage to live as a unicellular pathogen Rebbeck CA et al. [64]. Despite a massive substitution burden, these mammalian somatic cells were able to survive unchanged for millennia Murchison et al. [65].
The coital way of transmission is also used by human cancer. Unlike the canine venereal tumor, the transmission of human cancer is performed not by tumor cells but by the genomic predecessors of the cancerous cells: the gametes Rumyantsev [8] and Rumyantsev [66]. The invasive agents of human cancers go through many remarkable adaptations that enable this mammalian cell lineage to live as a unicellular pathogen of mammalian origin.
At the same time, in contrast to animal cancer, the malignant disease of humans does not possess the ability to transfer its living cells from one person to another. Human cancer ensures the maintenance of its own life before its prey is exhausted and dies in another way. The transmission of human cancer proceeds in the form of a cancer programmed genomic component as an intruder in the genome of the diseased person. In evolution, human cancer developed its transmission by means of a very regular way of communication between victims: sexual intercourse. The transfer of human cancer is also vector-mediated. Functions of vectors may be performed either by male or female gametes. During the formation of the intruder zygote, the deviant components of the xenogamous genetic code appear to be included in the united genetic code. The components continue to exist in it and function over the creation of intruded cells with their plethora of both banal and unique traits. Once implanted in the genome of its current host, human cancer ensured that it would be reproduced in the genomes of children via self-reproduction of the cancer-carrying parent. Like other components of genetic code, they were able to reproduce in the descendant genomes and thus multiply and disperse between people.
In contrast to canine venereal tumor, the genomes of most human cancers, with them between 1000 and 5000 somatic substitutions, contain several hundred times fewer somatic alterations. Some 20 distinct alteration signatures were found in many cancer types whereas others were confined to a single cancer class. Hypermutation, localized to small genomic regions or ‘kataegis,’ was found in many cancer types Alexandrov et al. [67]. The results reveal the diversity of substitution processes underlying the genesis of cancer, with potential implications for understanding cancer etiology. The genomic carrier of human cancer can be deposited either in male or female gametes. It is characterized by a complex of traits necessary for providing the ability of precursor prey to transmit cancerous genomes into relevant gametes, execute multifold acts of fertilization and breed the descendants (cancerous units) to the stage usually called complete maturity. The absence of any of these abilities sharply diminishes the chances of the cancerous genome prolonging its life in the genomes of descendant generations [68].
Conclusion
The above investigation was devoted to the further development of a principally new paradigm of cancer origin, pathogenesis and epidemic spread based on the hypothesis of carcinogenic transformation of reproductive genomes. The newly performed updates to the paradigm were based on multidisciplinary integrative reassessment and re-sensing of both well-known and recent data about cancer epidemiology, immunology, genetics, pathogenesis and clinical manifestations from the viewpoint of up-to-date, all-pathological, immunological, genetic, anthropological and evolutionary discoveries [69].
The reconsideration presented the actual data regarding cancer origin and current subsistence from the viewpoint of recent allpathological, epidemiological, immunological, clinical, genetic, and evolutionary discoveries allowed the formation of a new integrative paradigm - the hypothesis of genome intrusion - about the origin and pandemic spread of the disease. The main pioneer conclusion of performed discovery is that human cancer is a foreign biological entity adapted, in it evolution, to invade inside of human body and exist in it at the expense of stuffs and functions of the intruded organism. Cancerous disease is a result of ecological functions performed by the subsistence of cancer inside of invaded human body. The circle of cancer life was also figured in the paradigm for the first time. Except some unique biological traits, mainly those providing it with the ability to invade victim, reproduce in and be transmitted to the bodies of new prays cancer may be considered as analogous to the plethora of parasites existed in the world [70].
The revealed evidence allowed the most exhaustive presentation of the invasive hypothesis of cancer existence as follows:
1. The evolutionary emergence of cancer was predetermined by genome transformations that created, in evolution, inter- taxon differences in the molecular constitution of inherent physiological systems responsible for the regulation of cell dividing and tissue growth.
2. The development of individual cancer is initiated by the appearance in the afflicted body of a deviant cell clone (or clones) inherently immune to the normal physiological regulators of cell growth and tissue formation. The cells of such inherently immune clones are able to grow independently of physiological control of normal cell replication. These clones are foreign (alien, non-self) to the afflicted body but with many of its traits.
3. The inherently immune clones appear in a body as the result of xenogamy (genetic admixture) due to crossbreeding between parental gametes made up of partially different genotypes, which leads to both the intrusion of offspring’s genome with heterozygous genes and to the formation in the offspring’s body of coexisting cell clones with opposite relations to the regulators of growth. The currently observed increasing incidence of the disease depends on the intensity of xenogamous genetic admixture within ethnically mixed populations.
4. The emergence of cancerous clones and their dispersion around the body in the form of discrete micro-populations are performed before postnatal ontogeny in the manner used in the dispersion of other embryonic tissues and organs. Thus, the lymphatic system of individual adaptive immunity does not recognize the deposited cancer cells as foreign and does not destroy them.
5. After the end of their dispersion, the subpopulations reside in their places like cell masses of smaller but different sizes. At a relevant time of a host’s life (mainly after 40 years of its age), the uncontrolled growth of such micro-subpopulations become visible in the form of detectable extra cell masses of cancerous tissue, the multiple & evolutionary emer tumors. The largest of the subpopulations achieves a detectable tumor far earlier than the smaller ones, thus the first apparent cell mass is usually called the ‘primary’ tumor.
6. Cancerous cell populations subsist on life-supporting material provided by the infected organism. Any individual cancer arises and exists because of natural ecological relations between two organisms in which the xenogamous one (the consumer) obtains the energy for its life at the expense of substances composed of the consumed organism (the victim). Cancer is a kind of parasitism.
7. The marauding way of life exploited by populations of cancerous cells is performed mainly by their molecular enzymatic agents targeting either the splitting of the host’s macromolecules or produced inhibition of the victim cells.
8. The growth of all subpopulations of a cancerous clone is controlled by their own united physiological mechanism which maintains the whole structure of cancer within its genetically predetermined size. The destruction of one or more tumors gives boost to growth of other subunits of the clone.
9. As a sexually-transmitted parasite, human cancer possesses a set of constitutional, adaptive, inherently immune traits that could be the result of evolution over many millennia. The date of its initiation could be referred, for instance, to the epoch of xenogamous intercourse of Homo sapiens with Homo neandertalensis.
10. The current pandemic spread of cancer has been brought about the growing expansion of inter-ethnic admixture favored by growing industrialization, urbanization, globalization, and migration. Prevention of cancer could be achieved by voluntary restriction of xenogamous fertilization as well by orientation on non-cancerous genealogies.
The goal of this article was to perform the most exhaustive presentation of the hypothesis of xenogamous origin, parasite subsistence and pandemic spread of human cancer [71]. The focus is on the stages of cancer subsistence beginning from the initial invasion of the host’s genome by a cancerous gamete. Over the following mutual embryogenesis of both victim and intruder, the subsistence of the latter is supported by the inherent possession by the cancer cells of constitutive immunity to the host’s cell regulation management and immune defense. In many cases, cancer may consist of distantly separated units. The dispersion of cancerous units around the host’s body is performed over embryogenesis but before postnatal ontogenesis. The heterogenic make-up of any cancer is evidence of its source at the initial cancerous gamete [72].
The hypothesis is especially devoted to cancer subsistence at the expense of substances and energy derived from the body of its prey. This aspect of cancer subsistence is crucial for the progression of cancer within the host’s body. It is responsible for the pernicious impact of cancer on its victim. The existence of cancer as an autonomous living being can be also confirmed by the data about self-regulation of cancer physiology as well as physiological synchronization and communication between cancerous units. The transmission of cancer from one victim to another is performed through sexual intercourse via reproduction. Cancer is a kind of sexually-transmitted disease. The transmitted cancerous genome realizes it parasitic traits amongst the offspring and future generations.
These new notions provide the framework and some initial landmarks for the location of genomic roots of cancer origin and should encourage new research ideas and proposals for cancer prevention and therapy. Most advantageous are perspectives in the prevention of cancerous disease. There remains much to learn about this extraordinary unique and extremely complex disease. According to the paradigm, the search for a coveted clue to the genomic roots of cancer would be oriented on the discovery of structural and functional differences between the genomes of cancerous and normal cells [73].
“Will our children develop cancer?” This tough question should be asked by each couple before they decide to marry. The circle of cancer life will be inevitably repeated in every consequent generations of initially invaded parent. In fear of cancerous offspring people should chose spouses of non-promiscuous and noncancerous genealogy.
Appropriate genetic and genealogical evaluation must be performed before conception. First, the cancerous genealogies of expectant moms and dads must be discovered in detail. Their genomes must also be tested for the risk of cancer in their potential children. The results can provide early warnings about cancer, the deadliest disease. The warnings can help people to make rational decisions about their marital plan. This kind of protective parenting is now on its way to becoming a mainstream medical test. The hypothesis of xenogamous origin, parasite subsistence and pandemic spread of human cancer does not open optimistis perspectives for the healing of cancerous disease. All previous efforts of medicine in the chemotherapy but especially in the surgical and radiological healing of cancer appeared futile while they were not reasoned by the biology of cancer and pathogenesis of cancerous disease. What is more, the hypothesis lead to the discredit of surgical and radiological cure of cancerous disease. This negative opinion is argumented by activation of secondary tumors after resection of primary tumor.
References
- Pederson P (2011) On cancer and people. Science 332: 423.
- Stratton MR, Rahman N (2008) The emerging landscape of breast cancer susceptibility. Nature Genetics 40(1): 17-22.
- Marshall E (2011) Cancer research and the $90 billion metaphor. Science 331(6024): 1540-1541.
- Sharon Begley (2008) Rethinking the war on cancer. Newsweek.
- Anonymous (2011) 40 years of the war on cancer. Science 331(6024): 1540-1544.
- Rumyantsev SN (2008a) Cancer, hereditary immunity: Fundamental principles and exploitation in life study and health care, New York: Nova Biomedical Books, USA, pp. 210-212.
- Rumyantsev SN (2012) Toward the genomic roots of cancer. Journal of Medicine and Medical Sciences 3: 638-659.
- Rumyantsev SN (2013a) Human cancer is a parasite spread via intrusion in genome. Pure and Applied Biology 2(1): 7-16.
- Bauer KH (1928) Mutations theorie der Geschswulst-Entstehung. Berlin: Springer 16(62): 348.
- Rumyantsev SN (2009 b) The uniqueness and ordinariness of cancer origin and pathogenesis: new epidemiological, clinical and preventive perspectives. Journal of Clinical Medicine Research 1(1): 32-36.
- Rumyantsev SN (2010) Hypothesis: Toward the origin of cancer epidemics and pathogenesis. Journal of Carcinogenesis 9: 1-7.
- Rumyantsev SN (2011) Functions of hereditary immunity and xenogamy in cancer origin and pandemic spread. Open Journal of Immunology 1(2): 27-40.
- Rumyantsev SN (2009a) The discredit of cancer metastasis science advisory board.
- Rumyantsev SN (2008c) Phenomenon of Immune Mosaicism, Hereditary Immunity: Fundamental Principles and Exploitation in Life Study and Health Care, Nova Science Publishers, New York, USA, pp. 81-102.
- Rumyantsev SN (2010a) Bioecology of pleistocenic spurt in anthropogenesis. International Journal of Integrative Biology 10: 14-21.
- Rumyantsev SN (2008b) Hereditary immunity: fundamental principlesand exploitation in life study and health care. New York: Nova Biomedical Books, USA.
- Rumyantsev SN (2003) The intra-individual diversity in senescence. Biogerontology 4(3): 171-178.
- Rumyantsev SN, Gerasimov VK (2007) The Origin and functions of biodiversity in infectious and non-infectious diseases, focus on biodiversity research. In: Schwartz J (Ed.), Nova Science Publishers, USA, pp. 199-300.
- Mc Culloch M, Jezierski T, Broffman M, Hubbard A, Turner K, et al. (2006) Diagnostic accuracy of canine scent detection in early- and late-stage lung and breast cancers. Integrative Cancer Therapies 5(1): 30-39.
- Rumyantsev SN (1997) Chemical ecology and biomolecular evolution. Acta Biotheoretica 45(1): 65-80.
- Siegel R, Ward E, Brawley O, Jemal A. (2011) Cancer statistics, 2011: The impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer Journal for Clinicians 61(4): 212-236.
- Gilbert SF (2000) Developmental Biology, 6th edn Sunderland, Massachusetts: Sinauer Associates, Inc., Sunderland, Massachusetts, USA.
- Soerjomataram I, Coebergh JW (2009) Epidemiology of multiple primary cancers. Methods in Molecular Biology 471: 85-105.
- Levi F, Randimbison L, Te VC, Conconi MM, La Vecchia C (2008) Risk of prostate, breast and colorectal cancer after skin cancer diagnosis. International Journal of Cancer 123(12): 2899-2901.
- Milan T, Pukkala E, Verkasalo PK, Kaprio J, Jansen CT, et al. (2000) Subsequent primary cancers after basal-cell carcinoma: a nationwide study in Finland from 1953 to 1995. International Journal of Cancer 87(2): 283-288.
- Nugent Z, Demers AA, Wiseman MC, Mihalcioiu, Kliewer EV (2005) Risk of second primary cancer and death following a diagnosis of nonmelanoma skin cancer. Cancer Epidemiology, Biomarkers & Prevention 14(11 Pt 1): 2584-2590.
- Soerjomataram I, Louwman WJ, Lemmens VE, Coebergh JW, de Vries E (2008) Are patients with skin cancer at lower risk of developing colorectal or breast cancer? American Journal of Epidemiology 167(12): 1421-1429.
- Kreso A, O’Brien CA, van Galen P, Gan OI, Notta F, et al. (2013) Variable clonal repopulation dynamics influence chemotherapy response in colorectal cancer. Science 339(6119): 543-548.
- Hanahan D, Coussens LM (2012) Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 21(3): 309- 322.
- Hanahan D, Weinberg RA (2011) Hallmarks of cancer: The next generation. Cell 144(5): 646-674.
- Mardis ER (2012) Genome sequencing and cancer. Current Opinion in Genetics & Development 22(3): 245-250.
- Marusyk A, Polyak K (2013) Cancer. Cancer cell phenotypes, in fifty shades of grey. Science 339(6119): 528-529.
- De Wys WD, Malone WF, Butrum RR, Sestili MA (1986) Clinical trials in cancer prevention. Cancer 58 (8 Suppl): 1954-1962.
- Tisdale MJ (2003) Pathogenesis of cancer cachexia. Journal of Supportive Oncology 1(3): 159-168.
- Carmeliet P, Rakesh KJ (2000) Angiogenesis in cancer and other diseases. Nature 407(6801): 249-257.
- Ehrmann RL, Knoth M (1968) Choriocarcinoma: transfilter stimulation of vasoproliferation in the hamster cheek pouch studied by light and electron microscopy. The Journal of the National Cancer Institute 41(6): 1329-1341.
- Hanin L (2013) Seeing the invisible: how mathematical models uncover tumor dormancy, reconstruct the natural history of cancer, and assess the effects of treatment. Advances in Experimental Medicine and Biology 734: 261-282.
- Folkman J, Watson K, Ingber D, Hanahan D (1989) Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature 339(6219): 58-61.
- Prehn RT (1993) Two competing influences that may explain concomitant tumor resistance. Cancer Research 53(14): 3266-3269.
- Retsky M, Demicheli R, Hrushesky W, Baum M, Gukas I (2010) Surgery triggers outgrowth of latent distant disease in breast cancer: An inconvenient truth? 2(2): 305-337.
- Baum M, Chaplain M, Anderson A, Douek M, Vaidya JS (1999) Does breast cancer exist in a state of chaos? European Journal of Cancer 35(6): 886-891.
- Demicheli R, Retsky M, Hrushesky WJM, Baum M, Gukas ID (2008) The effects of surgery on tumor growth: a century of investigations. Annals of Oncology 19(11): 1821-1828.
- Hanin L, Korosteleva O (2010) Does extirpation of the primary breast tumor give boost to growth of metastases? Evidence revealed by mathematical modeling. Mathematical Biosciences 223(2): 133-141.
- De Jong KP, Lont HE, Bijma AM, Brouwers MA, de Vries EG, et al. (1995) The effect of partial hepatectomy on tumor growth in rats: in vivo and in vitro studies. Hepatology 22(4 Pt 1): 1263-1272.
- Garcia-Alonso I, Palomares T, Alonso A, Portugal V, Castro B, et al. (2003) Effect of hepatic resection on development of liver metastasis. Revista espanola de enfermedades digestivas 95(11): 765-770.
- Ikeda Y, Matsumata T, Takenaka K, Sasaki O, Soejima K, et al. (1995) Preliminary report of tumor metastasis during liver regeneration after hepatic resection in rats. European Journal of Surgical Oncology 21(2): 188-190.
- Elias D, De Baere T, Roche A, Ducreux M, Leclere J, et al. (1999) During liver regeneration following right portal embolization growth rate of liver metastases is more rapid than that of the liver parenchyma. British Journal of Surgery 86(6): 784-788.
- Von Schweinitz D, Fuchs J, Gluer S, Pietsch T (1998) The occurrence of liver growth factor in hepatoblastoma. European Journal of Pediatric Surgery 8(3): 133-136.
- Sorin V, Mizrahi A, Ohana P, Ayesh S, Birman T, et al. (2009) Partial hepatectomy in rats results in significant growth of liver metastases by increased expression of H19 gene. Cancer Therapy 7: 240-244.
- Giuliano AE, Hunt KK, Ballman KV, Beitsch PD, Whitworth PW et al. (2011). Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. Journal of the American Medical Association 305(6): 569- 575.
- Pockaj BA, Wasif N, Dueck AC, Wigle DA, Boughey JC, et al. (2010) Metastasectomy and surgical resection of the primary tumor in patients with stage IV breast cancer: Time for a second look? Annals of Surgical Oncology 17(9): 2419-2426.
- Lange PH, Hekmat K, Bosl G, Kennedy BJ, Fraley EE (1980) Accelerated growth of testicular cancer after cytoreductive surgery. Cancer 45(6): 1498-1506.
- De Giorgi V, Massi D, Gerlini G, Mannone F, Quercioli E, et al. (2003) Immediate local and regional recurrence after the excision of a polypoid melanoma: Tumor dormancy or tumor activation? Dermatologic Surgery 29(6): 664-667.
- Tseng WW, Doyle JA, Maguiness S, Horvai AE, Kashani-Sabet M, et al. (2009) Giant cutaneous melanomas: evidence for primary tumour induced dormancy in metastatic sites? British Medical Journnal Case Reports.
- Deylgat B, Van Rooy F, Vansteenkiste, Devriendt D, George C (2011) Postsurgery activation of dormant liver micrometastasis: a case report and review of literature. Journal of Gastrointestinal Cancer 42(1): 1-4.
- Skegg DCG, Corwin PA, Paul C, Doll R (1982) Importance of the male factor in cancer of the cervix. Lancet 2(8298): 581-583.
- Shyman S, Aganoal SS, Sehgal A, Sardana S, Kumar A, et al. (1993) Role of male behavior in cervical carcinogenesis among women with one lifetime sexual partner. Cancer 72(5): 1666-1669.
- Rosenblatt KA, Wicklund KG, Stanford JL (2001) Sexual factors and the risk of prostate cancer. American Journal of Epidemiology 153(12): 1152-1158.
- Sarma AV, McLaughlin JC, Wallner LP, Dunn RL, Cooney KA, et al. (2006) Sexual behavior, sexually transmitted diseases and prostatitis: the risk of prostate cancer in black men. Journal of Urology 176(3): 1108-1113.
- Werren JH (2011) Selfish genetic elements, genetic conflict, and evolutionary innovation. Proceedings of the National Academy of Sciences of the United States of America108 (suppl 2): 10863-10870.
- Giraldo Perez P, Goddard MR (2013) A parasitic selfish gene that affects host promiscuity. Proceedings of the Royal Society Biological Sciences 280(1770): 20131875.
- Burgasov PN, Rumyantsev SN (1974) Evolution of Clostridiosis [in Russian]. Meditsine, Moscow, Russia.
- Murgia C, Pritchard JK, Kim SY, Fassati A, Weiss RA (2006) Clonal origin and evolution of a transmissible cancer. Cell 126(3): 477-487.
- Rebbeck CA, Thomas R, Breen M, Leroi AM, Burt A (2009) Origins and evolution of a transmissible cancer. Evolution 63(9): 2340-2349.
- Murchison EP, Wedge DC, Alexandrov LB, Fu B, Martincorena I, et al. (2014) Transmissible dog cancer genome reveals the origin and history of an ancient cell lineage. Science 343(6169): 437-440.
- Rumyantsev SN (2013b) Human cancer is transmitted via genome. Open Journal of Genetics 3(1): 6-11.
- Alexandrov LB, Nik Zainal S, Wedge DC, Aparicio SA, Behjati S, et al. (2013) Signatures of mutational processes in human cancer. Nature 500(7463): 415-421.
- War on Cancer is a Dismal Failure (2010) Natural News com 1999-2007 Cancer Incidence and Mortality Data. 2007. National Program of Cancer Registries. Betesda, Maryland, USA.
- Albiges L, Molinie V, Escudier B (2012) Non-clear cell renal cell carcinoma: does the Mammalian target of rapamycin represent a rational therapeutic target? Oncologist 17(8): 1051-1062.
- Briasoulis E, Pavlidis N (1997) Cancer of unknown primary origin. The Oncologist 2(3): 142-152.
- Greenblatt M, Shubik P (1968) Tumor angiogenesis: Trans filter diffusion studies in the hamster by the transparent chamber technique. The Journal of the National Cancer Institute 41(1): 111-124.
- Quintana E, Piskounova E, Shackleton M, Weinberg D, Eskiocak U, et al. (2012) Human melanoma metastasis in NSG mice correlates with clinical outcome in patients. Science Translational Medicine 4(159): 159ra149.
- Van de Stolpe A, den Toonder JMJ (2014) Circulating tumor cells: what is in it for the patient? A vision towards the future. Cancers 6(2): 1195- 1207.
© 2018 Sergey N Rumyantsev. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






