- Submissions

Full Text
Novel Approaches in Cancer Study
Advances of Cancer Synergic Photo-Therapy: Kinetics and Efficacy
Jui-Teng Lin*
Chairman & CEO of New Vision Inc Taipei, Taiwan
*Corresponding author:Jui-Teng Lin, Chairman & CEO of New Vision Inc Taipei, Taiwan
Submission: July 17, 2018;Published: August 28, 2018

ISSN:2637-773XVolume2 Issue1
Abstract
The kinetics and efficacy of anti-cancer via phototherapy are reviewed. Factors influencing the cancer therapy efficiency in both photothermal therapy (PTT) and photodynamic therapy (PDT) using nanogold particles and photosensitizers (PS), respectively, are analyzed. Efficacy of cancer therapy may be enhanced by combining PTT and PDT either activated by one light or two lights. For maximum PTT/PDT synergistic efficacy, the concentration of photosensitizers and nanogold required optimization, besides the wavelength of the light matching the absorption peak of PS and nanogold, and the sequential order of PTT and PDT process. External supply of either photosensitizers or oxygen concentration will significantly improve the anti-cancer efficacy via type-II PDT. The singlet oxygen threshold dose for PDT and cell viability are governed by the product of PS concentration and light dose.
Keywords: Photothermal therapy; Photodynamic therapy; Optimal; Synergistic effect; Modeling; Heat diffusion; Photochemical kinetics
Introduction
A recent review article of Yurt & Tuncel [1] reported the clinical aspects of combined photodynamic and radiotherapy synergistic effect in cancer treatment. Synergic method combining photothermal therapy (PTT) and photodynamic therapy (PDT) using nanogold particles and photosensitizers were also reported [2-4]. Besides the synergic methods, improvement of the efficacy of anti-cancer also include the use of nano-medicine and nanoparticles [5]. The efficacy of PDT may be further improved significantly via conjugated nanogolds. For example, it was reported by the conjugated spherical nanogold as the delivery agent for 5-ALA resulted in a two times higher cell death rate compared to free 5-ALA [6]. Another example is that the DNA damage caused by PDT as demonstrated by alkaline gel electrophoresis was greater in the methylene blue (MB) plus chitosan-treated group than in control and MB-treated groups [7,8].
Cancer or tumor cells death may be caused by photothermal ablation, mechanical damage, and increase in the localized drug concentration. Gold nanoparticles are promising agents for cancer therapy, drug carriers, photo-thermal agents and contrast agents. The U.S. FDA has approved numerous Investigational New Drug (IND) applications for nano-formulations, enabling clinical trials for breast, gynecological, solid tumor, lung, mesenchymal tissue, lymphoma, central nervous system and genitor-urinary cancer treatments. Comparing to the visible light, near-infrared (NIR) light offers the advantages of larger absorption and scattering cross sections and much deeper penetration depth in tissues [9-12].
The red-shift of the absorption peak in nanorods is governed by the aspect ratio (defined by as the ratio of the length to the crosssectional diameter), whereas it is governed by the shell thickness in Nano shells [12]. Recent studies have shown that gold nanorods conjugated to antibodies [11-13] could be used for selective and efficient photothermal therapy. Lin et al. [10] proposed the use of a diode laser system having multiple wavelengths for more efficient treatment of cancer tumor. To overcome the penetration issue, Lin et al also proposed the use of a train-pulse to increase the volume temperature increase [10] which is particularly useful to larger volume tumors, unless an inserting fiber is used to deliver the laser energy.
New synergistic treatment modalities combining PDT with PTT could overcome current limitations of PDT, thus achieving enhanced anticancer efficacy. To promote the tumor accumulation of photosensitizers (PS) and to generate heat for synergistic PDT/PTT [10], surface conjugation of PS on nanoparticles has been proposed, which however, has limitations including relatively low loading capacity and the possible leakage of PSs coupled on nanoparticle surfaces during their circulation in biological systems. In this study, we will review the factors influencing the cancer therapy efficiency in both PTT and PDT using nanogold particles and photosensitizers, respectively. The fundamental and kinetics of both type-I and type- II PDT will be presented by comprehensive modeling and analytic formulas. The in vitro singlet oxygen threshold dose at PDT will be discussed.
Methods
Efficacy of PDT
As shown by Figure 1, factors influencing the efficacy of PDT include: selectivity, penetration and optimization, where maximum light penetration depth and efficacy, minimum dose (or treatment time), and high selectivity are desired. Optimal combination of light energy (dose), intensity and irradiation time may be achieved via Lin-scaling laws, Arndt-Schulz-Law (for therapeutic window) and Bunsen-Roscoe law (for reciprocity rule) [13]. Bunsen-Roscoe law (BRL) of reciprocity stating that the effect of a photo-biological reaction is proportional only to the total irradiation fluence (or light dose) (E=It), or the product of intensity (I) and exposure time (t). To achieve the same efficacy, the required exposure time based on BRL is given by t=E/I. Based on BRL, treatment time may be shortened by using a higher intensity while maintaining the similar efficacy. However, BRL is still controversial and has limited validation, as reported by Lin [13].
Figure 1:Summary of factors influencing the efficacy of PDT for anti-cancer.
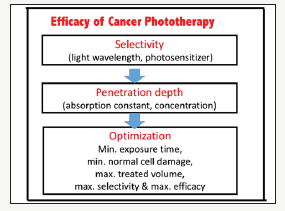
Effort to minimize complications, various modified PDT protocols have been explored involving reduced photosensitizer dosage, laser fluence, or a combination of both. Half-fluence, halfdose, half-fluence and micropulse, 1/3 dose, and minimal-fluence protocols have all demonstrated some degree of treatment effect [14,15]. Oxygen plays a critical role in the efficacy of Type-II PDT [16-21], where oxygen consumption and diffusion effects in PDT was first reported by Foster et al. [16] in 1991 and was updated and reviewed recently by Zhu et al. [19] in 2017. The kinetics of both oxygen-mediated (type-II) and non-oxygen-mediated (type-I) was reported by Lin recently for corneal crosslinking [20].
The kinetics of PDT
Figure 2:The kinetics of PDT showing both type-I and type-II pathways [20,21].
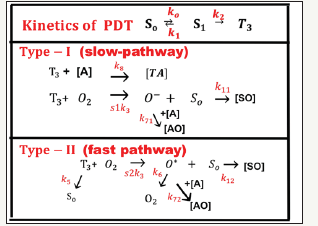
Figure 2 shows the kinetics of PDT for both type-I and type- II pathways [20]. PDT makes use of photosensitizers (PS) to generate reactive reactive species upon the absorption of specific wavelengths of light, where the selectivity is given by: (i) PSs are preferentially taken up by tumour tissues, and (ii) the molecules generate cytotoxic radical species only at the site where light is administered. There are two cytotoxic photochemical mechanisms in PDT (as shown by Figure 1): (i) Type-I mechanism where the molecule directly reacts through its triplet excited state to generate reactive radical’s species; and (ii) Type -II mechanism where PSs convert molecular oxygen into highly reactive singlet oxygen. Most PSs currently used in the clinic are predominantly oxygenmediated Type -II molecules. It is also possible that both Type-I and -II coexist.
Depending on the target site, PDT effects include destruction of blood vessels, killing of tumour tissue and cells, and induction of immune response. If the PS is also mainly retained in the blood vessels, the type-II process produced singlet oxygen (SO) can damage the blood vessels, causing insufficient blood supply to the lesion, indirectly cause cell death. When the PS reaches the cell, SO may lead to cell apoptosis, necrosis and autophagy. The path of death depends on the concentration and distribution of SO in the course of treatment. In addition, many studies have shown that PDT for tumor cells itself has a strong immunogenicity and can stimulate the specific immune response, where the patient’s active immune response to the tumor and can be automatically removed without irradiation.
Most PS available for PDT utilizes Type II photodynamic processes, i.e., the photodynamic effect is achieved through the production of singlet oxygen [16-20]. As shown in Figure 1, the process begins with the absorption of a photon by PS in its ground state, promoting it to an excited state. The PS molecule can return to its ground state by emission of a fluorescence photon or convert to a triplet state which may undergo a collisional energy transfer with ground state molecular oxygen (type II process), or with the substrate/target (type I process). In type II interaction, the PS returns to its ground state, and oxygen is promoted from its ground state (a triplet state) to its excited (singlet) state. In type- II process, the PS is almost not consumed (due to the slow singlet oxygen quenching rate), whereas in type-I process the PS is largely depleted specially for high intensity [20].
The Synergic system
As shown in Figure 3, the tumor cells killing efficiency may be enhanced by combining PTT and PDT using two light sources (either lasers or LED sources), in which the treated tumor tissue is injected by both nanogold solution and photosensitizers [2]. Depending on the types of photosensitizers and the shapes of the nanogold, the light wavelengths matching the absorption may vary from UV, visible to near IR (NIR). For examples, nanosphere absorbs visible light (at 480-680nm), nanocube (700-900nm), nanorod (700-2500nm), and nanoshell (480-810nm) [2].
Figure 3: Combined PTT and PTT processes using various lights having wavelength from UV to IR with associate nanogold shapes and photosensitizer [2].
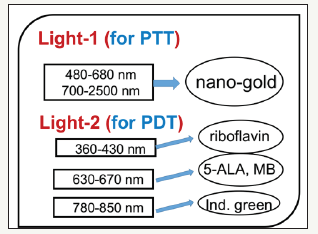
As shown in Figure 3, the combined PTT and PTT processes using various lights having wavelength from UV to IR with associate nanogold shapes and photosensitizer. Photosensitizer riboflavin (B2), 5-ALA, methylene blue (MB) and indocyanine green absorb, respectively, light at wavelength of (365, 430nm), (530-670nm), (780-850nm), as shown by Figure 3. Therefore, a combined dualfunction of PTT/PDT can be performed by: (i) an NIR light at NIR absorbed by gold nanorod and indocyanine green; or a visible light absorbed by gold nanosphere and 5-ALA; (b) two different lights having wavelength at NIR (for PTT) and UV to visible light (for PDT). For the case of one light for both PTT and PDT the simultaneously interacting with the nanogold and the photosensitizer is much more complex than that of the case of two different lights which can be treated independently.
The combined PTT/PDT efficacy
PDT process utilizes reactive oxygen species (ROS) generated through the reaction between photosensitizer (PS) and oxygen presented in tissues upon the irradiation of light to achieve effective treatment. The ROS is generated under a so-called type- II photochemical reaction which requires oxygen. In comparison, type-I process does not need oxygen and the triplet PS state can interact directly with the target for anti-cancer. Using the same light for both the PS and nanogold interaction is much more complex than when two light with different wavelengths are absorbed respectively by the PS and nanogold, in which the PTT and PDT can be treated independently.
The synergic effects of PTT and PDT, using two lasers at 808nm and 660nm, respectively, and nanogold in C6 gel, was reported by Kim et al. [3]. They reported higher efficacy when conduct PDT prior to PTT than [PTT+PDT]. This sequential-dependent process may be realized by that PTT may cause reduction of the kinetic constant and quantum yield due to increased temperature due to PTT, besides the potential reduction of oxygen supply which is critical in type-II PDT [2,3].
Optimal efficacy in PDT
For PDT, both type-I and type-II reactions occur in the photochemical reaction. The anti-cancer efficacy is related to the S-function by Ceff=1-exp(-S), where S1 (for type-I) and S2 (for type- II) are given by [20]
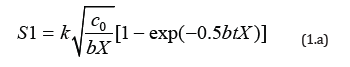
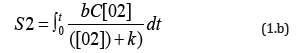
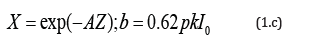
where C0 is the initial PS concentration (at z=0), I0 is the initial light intensity; [O2] is the concentration of oxygen. K and k are rate constant, and p is the PS triple state quantum yield. We have numerically showed that S1 has an optimal z*, whereas S2 is a decreasing function of z, and achieves a steady state in time. It was reported that type-II, or S2, is the predominant process for anticancer, which is governed by the oxygen concentration. S2 reaches a steady state in time when oxygen is completely depleted [19,21].
As shown by Equation (1.a), the S formulas show that S1~[C0/ I0]0.5, with no contribution from oxygen [O2]; whereas S2~[O2] C requires both C and [O2]. Therefore, resupply of PS or oxygen would improve the generation of ROS and improve the anti-cancer efficacy via type-II PDT. Figure 4 shows numerically produced typical profiles of oxygen and PS concentration, efficacy, singletoxygen, and the cell viability, for various light intensity of 50, 100, 200mWcm2, without external oxygen source (or P=0). With the supply of external oxygen (or P>0), one may improve the type-II efficacy (or S2 function).
Figure 4:The temporal profiles of: (A) oxygen (red curves) and PS concentration (blue curves), (B) S2-function, (C) cell viability, (D) efficacy vs. time, (E) singlet-oxygen, and (F) efficacy vs. light dose (E0), for various light intensity of 50, 100, 200mW/cm2, (for curves 1,2,3), without external oxygen source.
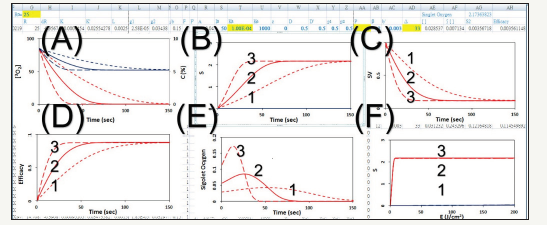
Figure 4 shows the following important features of type-II anticancer efficacy:
a. Higher light intensity has a faster depletion of oxygen and PS concentration;
b. For the same dose, higher light intensity has a faster rising curve, but all reach the same steady-state efficacy (and S-function) for type-II PDT; in contrast to type-I, in which higher light intensity has lower steady-state efficacy;
c. Cell viability is lower for high intensity, which also produces higher rate of singlet oxygen, but less accumulated value, as shown by Figure 4.
As shown by Figure 4 (D) and (F), for transient state, with bt<< 1, S2 (at z=0) = aqEC0[1-(k/X00)], so that S2 is proportional to the product of C0E0, and follows the linear BRL of reciprocity; (b) for large time, S2 is a nonlinear function of C0E0, and is given by S2=aqY [a1-a2Y- a3Y2], with Y= C0E0. The transient S2 also defines the threshold of cumulated singlet oxygen concentration, defined by when cell viability, CV=exp(-S2)< 0.36, or S2>S2*=1.0, which defines the threshold product of aqEC0[1-(k/X00)] =1.0, or [C0E0]*> [1+(k/X00)]/(aq). Therefore, larger C0 and/or X0, has a lower E0* and vice versa.
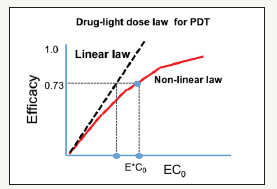
Figure 5 shows the efficacy versus product [EC0] showing the linear Bunsen-Roscoe law (BRL, dashed curve) and nonlinear law (solid curve). Also show are the threshold products. In vitro singlet oxygen threshold dose at PDT has been reported by Zhu et al. [22] and Klimenko et al. [23]. However, they have only reported the numerical results comparing with the measured data, whereas Lin [21] reported the first analytic formulas.
Figure 5:The efficacy versus product [EC0] showing the linear Bunsen-Roscoe law (BRL, dashed curve) and nonlinear law (solid curve). Also show are the threshold products [14].
Conclusion
Efficacy of cancer therapy may be enhanced by combining PTT and PDT either activated by one light or two lights. For maximum PTT/PDT synergistic efficacy, the concentration of PS and nanogold required optimization, besides the wavelength of the light matching the absorption peak of PS and nanogold, and the order of PTT and PDT process. External supply of either PS or oxygen concentration will significantly improve the anti-cancer efficacy via type-II PDT, which is limited by the generation of ROS, or the depletion of oxygen and/or PS concentration.
References
- Yurt F, Tuncel A (2018) Combined photodynamic and radiotherapy synergistic effect in cancer treatment. Nov Appro in Can Study 1(2): 1-3.
- Lin JT, Chen KT, HW Liu (2018) Novel techniques for improving anticancer efficacy via synergistic phototherapy. Op Acc J Bio Eng & Biosc 2(1): 1-5.
- Kim JY, Choi W, Kim M, Tae G (2013) Tumor-targeting nanogel that can function independently for both photodynamic and photothermal therapy and its synergy from the procedure of PDT followed by PTT. Journal of Controlled Release 171(2): 113-121.
- Wang YH, Chen SP, Liao AH, Yang CY, Ru Lee C, et al. (2014) Synergic delivery of gold nanorods using multifunctional microbubbles for enhanced plasmonic photothermal therapy. Scientific Report: 5685.
- Lin JT, Chen KT, Liu HW (2017) Progress of nanotechnology for phototherapy: Fundamentals and Applications. Med Devices Diagn Eng.
- Mohammadi Z, Sazgarnia A, Rajabi O, Soudmand S, Esmaily H, et al. (2013) An in vitro study on the photosensitivity of 5-aminolevulinic acid conjugated gold nanoparticles. Photodiag Photodany Therapy 10(4): 382-388.
- Shirata C, Kaneko J, Inagaki Y, Kokudo T, Sato M, et al. (2017) Nearinfrared photothermal/photodynamic therapy with indocyanine green induces apoptosis of hepatocellular carcinoma cells through oxidative stress. Scientific Reports: 13958.
- Akens MK, Wise Milestone L, Won E, Schwock J, Yee AJ, et al. (2014) In vitro and in vivo effects of photodynamic therapy on metastatic breast cancer cells pre-treated with zoledronic acid. Photodiag Photodany Therapy 11(3): 422-433.
- Huang X, El Sayed MA (2010) Gold nanoparticles: Optical properties and implementations in cancer diagnosis and photothermal therapy. J Advanced Research 1(1): 13-28.
- Lin JT, Chiang S, Lin GH, Lee H, Wei Liu H, et al. (2012) In vitro photothermal destruction of cancer cells using gold nanorods and pulsed-train near-infrared lase. J Nanomaterials: 861385.
- Lin JT (2011) Nonlinear optical theory and figure of merit of surface plasmon resonance of gold nanorods. J Nanophotonics 5(1): 051506.
- Lin JT (2010) Scaling law and figure of merit of biosensor using gold nanoshells. J Nanophotonics 4: 049507.
- Lin JT (2018) Lin- Scaling-laws for optimal efficacy in photo-biological systems versus Arndt-Schulz-Law and Bunsen-Roscoe law. Med Devices Diagn Eng 3:120-13.
- L in JT (2018) Analysis of drug-light dose on the efficacy of photodynamic therapy of age related macular degeneration. J Ophthalm Studies 1(1): 1-4.
- Düzgören I, Ruhi MK, Gulsoy M (2017) Effect of different laser power densities on photobiomodulation of L929 cell line. Proc of SPIE 10417: 1041702.
- Foster TH, Murant RS, Bryant RG, Knox RS, Gibson SL, et al. (1991) Oxygen consumption and diffusion effects in photodynamic therapy. Radiat Res 126(3):296-303.
- Hu XH, Feng Y, Lu JQ, Allison RR, Cuenca RE, et al. (2005) Modeling of a Type II photofrin-mediated photodynamic therapy process in a heterogeneous tissue thantom. Photochem Photobiol 81(6): 1460-1468.
- Wang KKH, Finlay JC, Busch TM, Hahn SM, Zhu TC, et al. (2010) Explicit dosimetry for photodynamic therapy: macroscopic singlet oxygen modeling. Journal of Biophotonics 3(5-6): 304-318.
- Zhu TC, Kim MM, Ong YH, Penjweini R, Dimofte A, et al. (2017) A summary of light dose distribution using an IR navigation system for Photofrin-mediated pleural PDT. Proc SPIE: 10047.
- Lin JT (2018) Efficacy S-formula and kinetics of oxygen-mediated (type-II) and non-oxygen-mediated (type-I) corneal cross-linking. Ophthalmology Research 8(1): 1-11.
- Lin JT, Chen KT, Liu HW (2018) Analysis of kinetics and efficacy of anticancer via oxygen-enhanced photodynamic therapy. J Cancer Research update 7(1): 21-26.
- Zhu TC, Kim MM, Liang X, Finlay JC, Busch TM, et al. (2015) In-vivo singlet oxygen threshold doses for PDT. Photonics Lasers Med 4(1): 59-71.
- Klimenko VV, Shmakov SV, Kaydanov, Knyazev NA, Kazakov NV, et al. (2017) In vitro singlet oxygen threshold dose at PDT with Radachlorin photosensitizer. SPIE Proc 10417.
© 2018 Jui-Teng Lin. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






