- Submissions

Full Text
Novel Approaches in Cancer Study
Multidisciplinary Approach to Prostate Cancer and Changes in Treatment Decision when Compared to Single Provider
Wenzler DL*, Blum E, Edwards L, Parslow J, Hollander JB, Wojno K, Krauss D and Korman HK
Michigan Urological Institute, USA
*Corresponding author:Wenzler DL, Beaumont Health System, Michigan Urological Institute, 3601 W 13 Mile Rd, St 438, Royal Oak, MI 48073-6769, USA
Submission: June 22, 2018;Published: July 18, 2018

ISSN:2637-773XVolume1 Issue5
Abstract
Introduction: In order to demonstrate the impact of multi-disciplinary care in the community oncology setting, we evaluated treatment decisions following the initiation of a dedicated genitourinary multi-disciplinary clinic (GUMDC).
Methods: In March 2010, a GUMDC was created at Beaumont Health System with the goal of providing patients multi-disciplinary evaluation and consensus treatment recommendations in a single visit. Urologists, radiation and medical oncologists along with ancillary support staff participated. The impact of the GUMDC on patient treatment decisions was analyzed and compared to decisions made by patients who were not referred to the GUMDC.
Results: From March 2010-12, a total of 98 men were evaluated for newly diagnosed prostate cancer in the GU MDC. This cohort was compared to 245 men in a private practice office. For all stages of prostate cancer, men seen in the GU MDC more often chose radiation, cryotherapy, and multimodal therapy. Men seen in the GU MDC less often chose active surveillance (AS), radical prostatectomy (RP), and hormonal ablation (HAT).
Conclusion: The establishment of a GU MDC improved the quality of care for cancer patients as demonstrated by improved adherence to NCCN guidelines.
Abbrevations: GUMDC: Genitourinary Multi-Disciplinary Clinic; AS: Active Surveillance; RP: Radical Prostatectomy; HAT: Hormonal Ablation; AUA: American Urological Association; NCCN: National Comprehensive Cancer Network; GUMDC: Genitourinary Multidisciplinary Clinic; NN: Nurse Navigator
Introduction
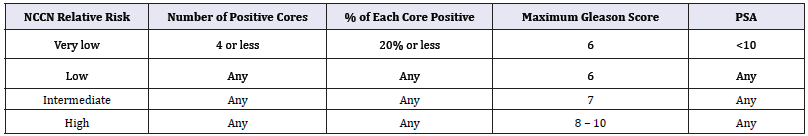
Prostate cancer is the most commonly diagnosed cancer and the second most common cause of cancer death in men, according to 2016 estimates [1]. Fortunately for patients, there are a multitude of treatment options for prostate cancer.Low grade prostate cancers can be indolent, giving patients the option to “watch” the cancer to avoid side effects of treatment. In fact, the American Urological Association (AUA) now states that active surveillance is recommended in patient with very low risk prostate cancer [2]. Patients who wish to pursue treatment also have options; these are primarily surgical removal (radical prostatectomy), radiation (in several forms) with or without hormonal ablation, hormonal ablation alone, or cyroablation. The National Comprehensive Cancer Network (NCCN) devised a risk stratificiation system in 2012, which was modified in 2016 [3]. This defines patient’s relative risk based upon number of positive biopsies, the amount positive in those biopsies, Gleason score and PSA. NCCN relative risk is shown in Table 1. The NCCN then further makes recommendations for treatment. These treatment recommendations are not just based on relative risk but also life expectancy. Other factors, such as quality of life, medical comorbidities, and erectile and urinary function should likely be considered as well. All of these factors require clinician judgement.
Table 1: NCCN relative risk.
Many patients have a plethora of treatment options to choose from when diagnosed with prostate cancer. Full discussion of treatment options can be overwhelming for the patient and families. Adding to this complexity is that different treatment options are administered by different specialists. Patients often require multiple office visits in multiple locations prior to making their treatment decision. This in inconvenient and leads to significant expense, as one study showed that prostate cancer is the second most expensive malignancy [4]. Furthermore, treatment can be based upon physician or institution preference or availability of treatment modalities and not necessary recommendations provided by organizations such as the AUA or NCCN [2-3]. We sought to reduce the burden on patients by creating a genitourinary multidisciplinary clinic (GU MDC). This allows patients to see all possible disciplines that would treat them for prostate cancer and get a full explanation of all available treatment options. The multidisciplinary approach has been shown to lead to excellent patient satisfaction and outcomes, and is has become a preferred practice in parts of the United States and Europe [5-6]. A previous study showed increased adherence to NCCN guidelines in patients seen in the GU MDC as opposed to those seen before its inception [7]. For this study, thefollowing question was posed: would a patient’s treatment decision changeif they were seen by all treating providers (as in the GU MDC) as opposed to by only a single provider (urologist)?
Materials and Methods
The Beaumont Health GUMDC was started in 2010. The structure of the clinic has been described in previous publication [7]. Briefly, following referral to the GU MDC, an intake form is generated by the nurse navigator (NN) and the patient’s case is presented at a multidisciplinary tumor board. A rapid response form is created with the treatment recommendations of the tumor board. The patient is greeted by the NN, who also takes a history, including standardized questionnaires. The patient then visits each medical specialty in their respective clinic in a series of coordinated consultations. Each specialist marks on the rapid response form if they are in agreement with the tumor board recommendation or any additional notes. After the last visit, a final consensus recommendation is made and a final recommendation is sent to the referring physician and primary care doctor. The NN coordinates scheduling of any additional tests or appointments. Subjects were identified through a query of electronic medical records of a single large private practice group containing 13 urologists. All patients seen with a new diagnosis of prostate cancer (ICD 9 code 185) from January 2010 through December 2012 were identified. The patient charts were then retrospectively reviewed. Data was collected on patient age, NCCNrecurrence risk, Charlson comorbidity index, and what treatment they received. For purposes of this study, the very low and low risk groups were combined and assigned the “low risk” label. Similarly, all radiation treatments were given the label “radiation” to reduce the available treatment options. The patients were then sorted into two groups: those that only saw a single provider (urologist) and those that were referred to the GU MDC. Differences between the two groups were evaluated and compared. A p-value < 0.05 was determined to be statistically significant.
Results
Table 2: Patients seen at each site stratified by NCCN recurrence risk. Age and Charlson comorbidity indeces were also compared between patients seen in both groups.
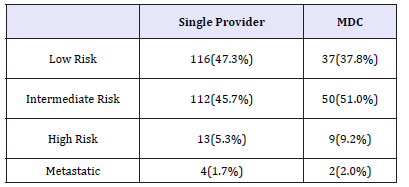
Table 3: Patients seen at each site stratified by recurrence risk and age.
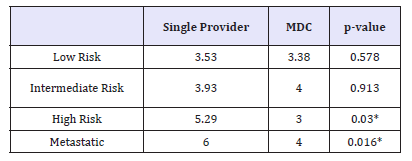
*=denotes statistically significant
Table 4: Patients seen at each site stratified by recurrence risk and Charlson comorbitiy Index

*=denotes statistically significant
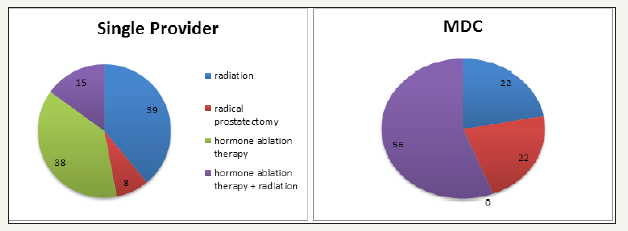
Between January 2010 and December 2012, 343 patients were diagnosed with prostate cancer in our group. Of these, 245 (71.4%) patients were seen by a single provider and 98 (28.6%) were seen in the MDC prior to choosing a treatment. Table 2 shows the patients broken down by NCCN recurrence risk. Age and Charlson comorbidity indeces were also compared between patients seen in both groups. Table 3 & 4 shows these data. Treatments chosen by the patient were also noted. In the low risk patients that saw one provider, 43% pursued radical prostatectomy, 41% pursued active surveillance, and 16% pursued radiation. Comparatively, these same patients that went to the MDC chose radical prostatectomy, radiation, and active surveillance 49%, 30% and 21% of the time respectively. These treatment options are shown in Figure 1. Many more treatments were pursued in the intermediate risk group. For example, those seen by a single provider chose radical prostatectomy most commonly (52%), followed by radiation (33%), active surveillance (6%), hormone ablation therapy (5%), hormone ablation therapy plus radiation (3%), and cryotherapy (1%). That same risk group that was seen in the MDC chose radiation and surgery most often (42% each), followed by hormone ablation plus radiation (6%), active surveillance and cryoablation (both 4%) and hormone ablation only (2%). These data are summarized in Figure 2. In the high risk group seen by a single provider, 39%, 38%, 15%, and 8% chose radiation alone, hormone ablation alone, hormone ablation and radiation, and radical prostatectomy, respectively. Of high risk patients seen in the MDC, the most common treatment choice was hormone ablation and radiation (59%), followed by radiation alone and radical prostatectomy (both 22%). A graphical representation of this is provided in Figure 3.
figure 1:Treatment choice of patients with low risk prostate cancer.
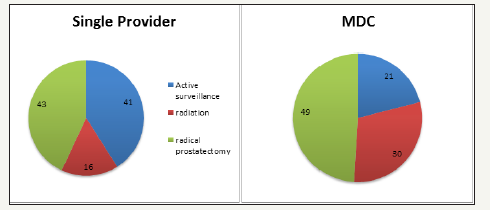
figure 2:Treatment choice of patients with intermediate risk prostate cancer.
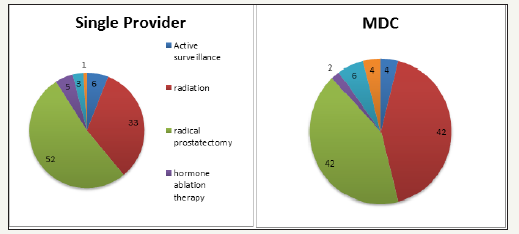
figure 3:Treatment choice of patients with high risk prostate cancer.
Discussion
Patients faced with a diagnosis of prostate cancer are faced with an equally daunting task of choosing a treatment. The GU MDC helps the care of prostate cancer be more convenient and expedited, and patients get a full explanation of all available treatment options from the specialists that deliver them. Multidisciplinary clinics have been used extensively for advanced cancers, as these often require specialists from many disciplines to manage the complexity of these diseases. Several groups have published the benefit of MDC in advanced prostate cancer [8-14]. This is intuitive because metastatic prostate cancer requires many disciplines, notably urology, medical oncology and radiation oncology. Fewer institutions have used multidisciplinary clinics for localized prostate cancer. Previous studies have shown that patients seen in MDC’s have increased adherence to guidelines for cancer care [7- 15]. Korman et al compared a 3 year window (2006-2008) in which patient saw a single provider to the GU MDC in 1 year 2010 [7]. While increased adherence to guidelines was noted, one downfall of this study was comparing different time periods. There is constant innovation in surgical devices, especially the robot (noted in the utilization of open prostatectomy from 14.6% in 2006 to 3.2% in 2008) and to the field of medicine in general that could alter which treatment patients prefer.
Other variables about multidisciplinary clinics have been examined, with various results. Stewart et al examined oncologic outcomes and wait times in patients seen in primary clinics compared to a GU MDC [16]. They found that more high risk patients were seen in the GU MDC than in the urology clinic, and patients had a shorter time from biopsy to radical prostatectomy. However, at follow up of 21 months, there was no difference in biochemical recurrence or adverse pathologic features between the two groups. The same group examined demographic information of patients being seen at their MDC [17]. They found these patients were more likely to be younger, Caucasian, have higher income and travel further for evaluation when compared to the local population. However, when patients elected treatment, these demographics shifted to be more consistent with the local population. Several studies have also shown changes in treatment outcomes over time. Kurpad et al showed that 22% of prostate cancer cases had a change in their practitioner-recommended treatment after being presented at multidisciplinary meetings [18]. However, this was lowest of the four major urologic cancers, , following bladder (44%), kidney (36%) and testicular (29%). In their impressive 15 year experience of an MDC, Gonnella et al reported improved survival of prostate cancer patients when compared to SEER data [19]. They also noted a migration toward robotic prostatectomy over the study period, which makes sense as innovation in surgical technology has markedly improved over that period of time.A shorter yet similar experience in Italy was reported by Magnani et al [20]. They noted high patient satisfaction, relatively frequent changes in treatment (up to 11%), stage migration to lower stage prostate cancer, and an increase in active surveillance over the six years. Similar to our study, this study showed that patients’ treatment changed when being seen in the GU MDC. Our study looked at several aspects of referrals to the GU MDC. First, we examined the stages that the patients were referred. We found that a higher proportion of patients were referred to the GU MDC who had intermediate (51.0% v 45.7%) and high risk (9.2% v 5.3%) prostate cancer. This intuitively makes sense as the risk of prostate cancer often requires more than one treatment or opinion, and it can benefit the patient to see more than just the urologist.
Second, we looked at age of patients at referral. We noted that of patients with intermediate and high risk cancer, the patients that were referred to the GU MDC were significantly younger. On average, intermediate risk patients seen by a single provider were 67.58 years old compared to 64.88 years old (p=0.0436). Even more striking was the difference in age with high risk patients: high risk patients seen by a single provider were 74.62 years old and those seen in the GU MDC were 58.78 years old (p=0.004).
Again, this tends to be common sense for most providers. Clinicians that treat prostate cancer are generally more aggressive with younger patients given the indolence of the disease. Given this, younger patients would typically be the best candidates for multimodality treatment that is delivered by several disciplines. Third, we examined Charlson comorbidity indices. Interestingly, the difference here were in patients with high risk and metastatic disease, and those seen in the MDC had lower scores (3.0 and 4.0 compared to 5.29 and 6.0, respectively, p=0.03 and 0.016). This seems counterintuitive. On further examination and thought, it can be easily explained. Patients with high risk and metastatic disease and high comorbidity indeces would likely just get hormone ablation as treatment, and therefore only be seen by a urologist who can administer this treatment. Healthier patients with high risk disease could be candidates for radiation or surgery, and those with metastatic disease could receive chemotherapy prior to hormone ablation.
Finally and most importantly, we looked at patient treatment choices and compared those patients who were seen by a single provider (urologist) and those seen by several providers in the GU MDC. This is not a new idea. Aizer et al examined the use of active surveillance in patients seen by a urologist and by a MDC [21]. They found more patients were treated with active surveillance and fewer with radiation and radical prostatectomy when seen in the GU MDC compared to a single provider. Older age, unmarried status, increased Charlson comorbidity index, fewer positive cores, and consultation at the GU MDC were significant predictors or patients choosing active surveillance. Regarding treatment decisions, our findings were contradictory to those noted by Aizer. We found that more low risk patients seen by the urologist only when compared to the GU MDC (41% compared to 21%). Similarly in the same low risk category, a much higher percentage underwent radiation when seen in the GU MDC when compared to single provider (30% v. 16%). A similar proportion of low risk patients pursued surgery (43% and 49%). In intermediate risk patients, the results were much less reproducible given the large number of treatments that patients underwent. A total of seven treatments were pursued by the intermediate risk patients. In the high risk and metastatic patients, many more patients underwent combination therapy (hormone ablation and radiation) in those seen in the GU MDC compared to single providers (56% compared to 15%). Surprisingly, no patients underwent hormone ablation as monotherapy when seen in the GU MDC. This is very different than those seen only by the urologist (38%). While no distinct conclusions regarding treatment decisions can be made from these data, we have several theories that may explain these differences in treatment. Regarding the decision of active surveillance, it may be that patients who elect to be seen in the GU MDC have a higher level of anxiety regarding their diagnosis, thus leading to more patients wishing to pursue treatment. With regards to more patients being treated with radiation when seen in the GU MDC, we feel this is likely due to having seen a radiation oncologist during their cancer consultations. When patients see the specialist that delivers the treatment, perhaps they get a fuller explanation of what it entails and possible outcomes, making them more comfortable with that treatment.
Our study has many advantages and new data. We directly compare patients seen by one group and by the MDC over the same time period. Comparing treatments over different time periods is a confounder, as treatments change over time. Previous data that younger and healthier patients are more likely to be referred for multiple opinions was confirmed in our study. However, we are the first study that we know of to show that AS is more common in those seen by a single provider. This is in direct contradiction to other studies, which have shown increased rates of AS in the GU MDC. Our study is not without limitations. First, the time period is short. It is unknown whether these trends would keep up over time. Second, all the “single providers” were in one group. The group itself may have their own practice and referral patterns, so the data may change if expanded to multiple groups. Finally, as mentioned previously, this sheds little light on why patients choose such treatments. Further work in this area is needed, and it could include a longer period of time, more patients and practices, or a survey study to determine patients’ motives for treatment.
References
- Cancer Facts and Figures (2016) American Cancer Society.
- Sanda MG, Chen RC, Crispino T, Freedland S, Greene K, et al. (2017) Clinically localized prostate cancer: AUA/ASTRO/SUO guideline. J Urol 534(17): 78003-78012.
- Mohler JL, Armstrong AJ, Bahnson RR, D’Amico AV, David BJ, et al. (2016) Freedman-cass DA, prostate cancer, featured updates to the NCCN guidelines. J Natl Compr Canc Netw 14(1): 19-30.
- Iadeluca L, Mardekian J, Chander P, Hopps M, Makinson GT (2017) The burden of selected cancers in the US: health behaviors and health care resource utilization. Cancer Manag Res 28(9): 721-730.
- Litton F, Kane D, Clay G, Kruger P, Belnap T, et al. (2010) Multidisciplinary cancer care with a patient and physician satisfaction focus. J Oncol Pract 6(6): e35-e37.
- Gomella LH, Lin J, Hoffman CJ, Dugan P, Guiles F, et al. (2010) Enhancing prostate cancer care through the multidisciplinary clinic approach: a 15- year experience. J Oncol Pract 6(6): e5-e10.
- Korman H, Lanni T, Shah C, Parslow J, Tull J, et al. (2013) Impact of a prostate multidisciplinary clinic program on patient treatment decisions and on adherence to NCCN guidelines. Am J Clin Oncol 36(2): 121-125.
- Basler JW, Jenkins C, Swanson G (2005) Multidisciplinary management of prostate malignancy. Curr Urol Rep 6(3): 228-234.
- Renzulli JF, Collins J, Mega A (2015) Radium-223 dichloride: Illustrating the benefits of a multidisciplinary approach for patients with metastatic castration-resistant prostate cancer. J Multidiscip Healthc 5(8): 279-286.
- Bolla M, Verry C, Giraud JY, Long JA, Conil M, et al. (2014) Results of a cohort of 200 hormone-naïve consecutive patients with prostate cancer treated with iodine 125 permanent interstitial brachytherapy by the same multidisciplinary team. Cancer Radiother 18(7): 643-648.
- Borso E, Boni G, Galli L, Ricci S, Farnesi A, et al. (2015) Radium 223 dichloride: a multidisciplinary approach to metastatic castrationresistant prostate cancer. Future Oncol 11(2): 323-331.
- Saad F, Sternberg CN (2007) Multidisciplinary management of bone complications in prostate cancer and optimizing outcomes of bisphosphonate therapy. Nat Clin Pract Urol 4(1): S3-S13.
- Taneja SS (2003) A multidisciplinary approach to the management of hormone-refractory prostate cancer. Rev Urol 5(2): S53-S59.
- Sternberg CN, Krainer M, Oh WK, Bracards S, Bellmunt J, et al. (2007) The medical management of prostate cancer: a multidisciplinary team approach. BJU Int 99(1): 22-27.
- Valicenti RK, Gomella LG, El-Gabry EA, Myers R, Nathan F, et al. (2000) The multidisciplinary clinic approach to prostate cancer counseling and treatment. Semin Urol Oncol 18 (3): 188-191.
- Stewart SB, Moul JW, Polascik TJ, Koontz BF, Robertson CN, et al. (2014) Does the multidisciplinary approach improve oncological outcomes in men undergoing surgical treatment for prostate cancer? Int J Urol 21(12): 1215-1219.
- Stewart SB, Banez LL, Robertson CN, Freedland SJ, Polascik TJ, et al. (2012) Utilization trends at a multidisciplinary prostate cancer clinic: initial 5-year experience from the Duke Prostate Center. J Urol 187(1): 103-108.
- Kurpad R, Kim W, Rathmell WK, Godley P, Whang Y, et al. (2011) A multidisciplinary approach to the management of urologic malignancies: does it influence diagnositic and treatment decisions? Urol Oncol 29(4): 378-382.
- Gomella LG, Lin J, Hoffman CJ, Dugan P, Guiles F, et al. (2010) Enhancing prostate cancer care through the multidisciplinary clinic approach: a 15- year experience. J Oncol Pract 6(6): e5-e10.
- Magnani T, Valdagni R, Salvioni R, Villa S, Bellardiat L, et al. (2012) The 6-year attendance of a multidisciplinary prostate cancer clini in Italy: incidence and management changes. BJU Int 110(7): 998-1003.
- Aizer AA, Paly JJ, Zietman AL, Nguyen PL, Beard CJ, et al. (2012) Multidisciplinary care and pursuit of active surveillance in low risk prostate cancer. J Clin Oncol 30(25): 3071-3076.
© 2018 Wenzler DL. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






