- Submissions

Full Text
Novel Approaches in Cancer Study
Lu-177 Dotatate External Dosimetry- An Update from 2013
Mushref Algarni1,2*, Robert Stodilka1, Janine Riffel1 and David Laidley1
1Department of Nuclear Medicine, London Health science, University Hospital, Schulich School of Medicine & Dentistry, Canada
2Department of Diagnostic Medical Imaging and Nuclear Medicine, King Fahd Military Medical Complex, Saudi Arabia
*Corresponding author: Mushref Algarni, Department of Nuclear Medicine, London Health science, University Hospital, Schulich School of Medicine & Dentistry, Ontario, Canada
Submission: September 15, 2017;Published: February 23, 2018

ISSN:2637-773XVolume1 Issue2
Abstract
London health sciences center is a frequent user of Lu-177 Dotatate for the treatment of neuroendocrine disease. We began using this radionuclide therapy as an in-patient procedure, where patients were released at 20 hours’ post therapy administration with minimal restrictions. Over the course of 2013, we worked to transform this therapy into an outpatient procedure, where patients were released at 4-6 hours’ post therapy administration with major restrictions.
The previous methods and data we presented in December 17, 2013 [1] were based on dose rates derived from cumulated doses measured over approximately 16 hours, and an assumption of Lu-177 clearance based on radioactive decay only.
Since 2013, our hospital has gained more experience with Lu-177 Dotatate, enabling us to develop a better understanding of dosimetry. We describe new dose measurements, a new model we developed to describe our observations, and a revised schedule of patient release and restriction duration.
Compared against previous measurements from 2013, our new measurements are of instantaneous (not cumulative) dose rates, and we now consider Lu-177 clearance to proceed both by physical decay and biologic excretion. Our new proposed model combines our experimental results with results from literature. The model will state the dose rate from time of discharge can be modeled as a decaying double exponential function.
Keywords: Dotatate; Dosimetry; Lu-177; Neuroendocrine; Lu-TATE
Abbreviation: LHSC: London Health Science Center; DRF: Dose Reduction Factor; EED: Estimated Effective Dose; EEDR: Estimated Effective Dose Received
Introduction
London Health Science Center (LHSC) is a frequent user of Lu-TATE for the treatment of Neuroendocrine Tumors NET. We began using this therapy as an in-patient procedure, where patients were released at 20 hours’ post therapy administration with minimal restrictions. Lu-TATE practices in Canada require patients to be placed on major restrictions for their first 20hrs after each dose administration. Therapy of Lu-177 clearance was based on radioactive decay only. Over the course of 2013, we worked to transform this therapy into an outpatient procedure, where patients were released at 4-6 hours’ post therapy administration with major restrictions [2-10].
Subjects and Methods
LHSC new proposed model combines our experimental results with results from literature. The model will state the dose rate from discharge can be modeled as a decaying double exponential function. The early phase clearance is dominated by protocoland patient-specific variables. Conversely, late-phase clearance is usually from disease-specific uptake. We designed an experiment to measure the dose reduction factor (DRF) due to patient selfshielding (R); the DRF due to patient voiding (V); and the four parametersλ1, λ2, α & β of our clearance model, CLR (t). Six patients were administered Lu-TATE. Activity range: 3700 - 5500MBq. Dose rate was measured at different time points post therapy [15-20].
Results
In six patients one hour pre-void & post-void, 5 hour & 20 hour mean dose rate was 5.6uSv/hr, 4.4uSv/hr, 2.28uSv/hr and 1uSv/hr respectively. We can determine dose reduction factor due to patient self-shielding (R) value which found to be 0.87. We determined fraction of radiotherapy product due to patient voiding by comparing pre- and post-void dose-rate measurements to be 0.87. Both predicted & experimental measurements dose rate for administered activities of 3700MBq at 1.9m plot with time data on the graph [21-25].
Discussion and Conclusion
In correlation with our previous data from 2013, we described an experiment where we measured cumulative doses from 14 patients. Dosimeters were placed in various locations in patients’ rooms and measured over different time periods. Our 2013 data shows variability and there are differences compared with our proposed model ranging from 3 - 32%. Overall, when we consider the 2013 results and our current model, predictions are reasonably close, especially considering differences may be due to the patients of 2013 moving around their hospital rooms. Our intention is to eventually use our new model to guide decisions for Lu-TATE patient restrictions and release from our institution.
Proposed Model
Clearance
Clearance processes are important to understand because they will allow us to predict dose rate from a radionuclide therapy patient after the patient is released from the hospital.
Clearance kinetics for many radiopharmaceuticals can be described as having an early phase and a late phase. Early phase clearance is often dominated by protocol- and patient-specific variables that influence non-specific uptake (for example: patient hydration, renal function). Conversely, late-phase clearance is usually from disease-specific uptake (for example: from a tumor). Many authors in literature model clearance kinetics using firstorder approximations (first order rate kinetics), resulting in clearance equations with decaying exponential functions. Following these examples, we propose the clearance of our radiotherapy product can be modeled as a summation of two exponential decay functions:
CLR t =α ×exp (−λ1×t) +β×exp (−λ2 ×t) [1]
Figure 1: In this figure, the radionuclide therapy is administered at time=0. At some point, the patient first voids their bladder, after which we begin to model the therapy product’s clearance kinetics. At first, clearance is rapid; and eventually clearance slows.
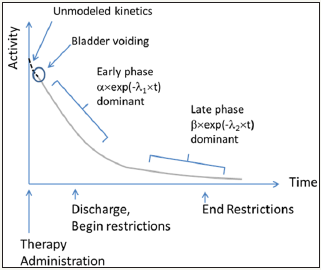
Where λ1 and λ2 describe the early phase and late phase effective decay constants, respectively; and α and β represent the respective proportions of our radiotherapy product governed by the early and late phase clearance processes. Note that the relationship between decay constant and half-life is λ=ln(2)/t1/2 where t1/2 is half-life.
We can show CLR (t) pictorially as: Figure 1
Dosimetry
The total estimated effective dose (EEDTOTAL) received by an individual in close proximity to the patient is proportional to the area under the patient activity vs time curve, from [time = discharge] to [time = infinity] weighted by patient restrictions. We express this as follows:
EEDTOTAL = EEDR (DS → RE) + EEDu (RE → ∞) + Di, [2]
Where DS = discharge time and RE = restrictions end time. EEDR is the estimated effective dose received by the caregiver from discharge to end of restrictions, during which restrictions are in effect. We define it as:
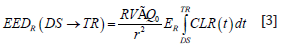
Where,
R [unitless] = dose reduction factor due to patient self-shielding (attenuation);
V [unitless] = dose reduction factor due to patient voiding after therapy administration;
Γ[uSvm2/MBqhr]= specific gamma constant for Lu-177;
Q0[MBq] = initial amount of Lu-177 administered to patient;
r [meters] = distance between patient and exposed individual;
ER [unitless] = restricted occupancy factor; and
t [hours] = time.
EEDU is defined the same as EEDR, except the restricted occupancy factor, ER, is replaced with the unrestricted occupancy factor, EU; and the limits of integration are [time = RE] to [time = infinity].
Integrating Equation 2 is straight-forward, but leads to a lengthy equation that we omit here for brevity.
The term Di accounts for dose to the caregiver arising from internalized radionuclide’s from the released patient, after NRC Regulatory guide 8.39. This is expressed as:
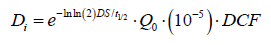
Where t1/2 is the Lu-177 physical half-life, 10-5 is the assumed fractional intake, and DCF is the dose conversion factor, taken as 0.05 [uSv/MBq].
What remains now is to assign numerical values to the variables. We have conducted an experiment to measure some of these values; whereas others are taken from literature, as will be described next.
Objective 2: Calculation of Voiding (V)
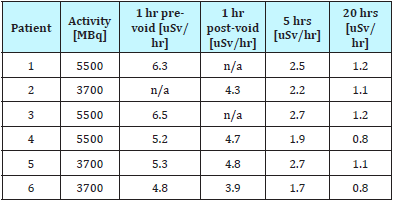
We determined the fraction of radiotherapy product in the patient after voiding by comparing pre- and post-void dose-rate measurements in Table 1. We found the average ratio to be 0.87. The corresponding value in literature is 0.54. This void ratio is highly dependent on protocol design and time of voiding. All of our patients were instructed to void 1 hour post-administration of therapy.
Table 1:
Objective 3: Parameters for clearance model CLR(t)
The following values for CLR(t) parameters: (Table 2)
Table 2:
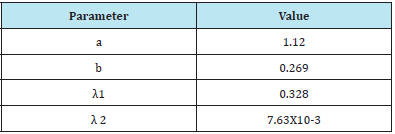
For interest, we note that the effective half-life associated with λ 1 is t1/2= 2.1 [hours], and λ 2 is t1/2=91 [hours].
Checking model again measurements
As a check of our results so far, we can plot our predicted dose rate for patients as a function of time; and compare this prediction with our measurements. Our predicted dose rate is given by:
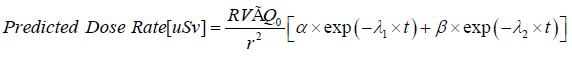
This predicted dose rate is compared with experimental measurements in Figure 2, for the case of 3700 MBq administered activities (Figure 2).
Figure 2

Model- Based Predictions of EED
In all cases, restrictions are assumed to result in 0.25 occupancy (Table 3).
Table 3:

Correlation with December 17 2013 Data
In our 2013 correspondence to you, we described an experiment where we measured cumulative doses from 14 patients. Dosimeters were placed in various locations in patients’ rooms and measured over different time periods (up to 20 hours’ post therapy administration). During this time, patients moved around the room: from their bed to the bathroom, for example.
We can compare this previous experimental data with our current model as follows:
In our previous study, the average activity administered was 5500MBq, and the maximum was 7400MBq.
We consider the longest cumulative dose integration time window in order to average patient movement
4-20 hours post therapy administration. The dosimetry location we will select is the bottom of the patients’ beds (near patient feet). We will take this location as approximately “1 meter” away from the patient.
The same parameters can be entered into our model: an activity of 5500 or 7400MBq, and a distance of 1 meter. We can use EEDR if we set DS=4 and RE=20, and restriction occupancy fraction to 1.
Below, shows the comparison of our 2013 data with our current mode. The corresponding calculations using our proposed model Table 4.
Table 4:

Scenario 8: From our 2013 data, the average dose measurement was 78uSv (assuming the average activity of 5500MBq). Our proposed model, assuming 5500MBq, yields a dose of 101uSv.
Scenario 8:From our 2013 data, we have 0.18uSv as a maximum dose (with 7400MBq administered activity), and 140uSv as the second highest recorded dose. Our model predicted 136uSv.
Our 2013 data shows variability, and there are differences compared with our proposed model ranging from 3% to 32%. Part of the variability and differences may be due to the patients of 2013 moving around their hospital rooms (their precise movement patterns over 20 hours were not monitored). However, overall we consider the 2013 experiment results and our model predictions reasonably close, especially considering we used two very different methods to arrive at dose.
Our intention is to eventually use our new model to guide decisions for Lu-177 Dotatate patient restrictions and release from our institution. We would appreciate your thoughts on our proposal.
References
- Olmstead C, Cruz K, Stodilka R, Zabel P, Wolfson R (2015) Quantifying public radiation exposure related to lutetium-177 octreotate therapy for the development of a safe outpatient treatment protocol Nucl Med Commun 36(2): 129-134.
- Fitschen J, Knoop BO, Behrendt R, Knapp WH, Geworski L (2011) External radiation exposure and effective half-life in Lu-177-Dota-Tate therapy. Z Med Phys 21(4): 266-273.
- Seregni E, Maccauro M, Coliva A, Castellani MR, Bajetta E, et al. (2010) Treatment with tandem [90Y] DOTA-TATE and [177Lu] DOTA-TATE of neuroendocrine tumors refractory to conventional therapy: preliminary results. Q J Nucl Med Mol Imaging 54(1): 84-91.
- Wehrmann C, Senftleben S, Zachert C, Müller D, Baum RP (2007) Results of individual patient dosimetry in peptide receptor radionuclide therapy with 177Lu DOTA-TATE and 177Lu DOTA-NOC. Cancer Biother Radiopharm 22(3): 406-416.
- Dauer LT, Boylan DC, Williamson MJ, St Germain J, Larson SM (2009) Clearance Kinetics and External Dosimetry of Iodine-131-labeled Murine and Humanized Monoclonal Antibody A33 in Patients with Colon Cancer: Radiation Safety Implications. Health Phys 96(5): 550-557.
- Barrington SF, Kettle AG, O’Doherty MJ, Wells CP, Somer EJ, et al. (1996) Radiation does rates from patients receiving iodine-131 therapy for carcinoma of the thyroid. Eur J Nucl Med 23(2): 123-130.
- Barrington SF, O’Doherty MJ, Kettle AG, Thomson WH, Mountford PJ et al. (1999) Radiation exposure of the families of outpatients treated with radioiodine (iodine-131) for hyperthyroidism. Eur J Nucl Med 26(7): 686-692.
- Guerriero F, Ferrari ME, Botta F, Fioroni F, Grassi E, et al. (2013) Kidney Dosimetry in 177Lu and 90Y Peptide Receptor Radionuclide Therapy: Influence of Image Timing, Time-Activity Integration Method, and Risk Factors. BioMed Research International 2013: 935351.
- Sandström M, Garske-Román U, Granberg D, Johansson S, Widström C, et al. (2013) Individualized dosimetry of kidney and bone marrow in patients undergoing 177Lu-DOTA-octreotate treatment. J Nucl Med 54(1): 33-41.
- Kwekkeboom DJ, de Herder WW, Kam BL, van Eijck CH, van Essen M, et al. (2008) Treatment with the radiolabeled somatostatin analog [177 Lu-DOTA 0, Tyr3] octreotate: toxicity, efficacy, and survival. J Clin Oncol 26(13): 2124-2130.
- Bodei L, Mueller-Brand J, Baum RP, Pavel ME, Hörsch D, et al. (2013) The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumors. Eur J Nucl Med Mol Imaging 40(5): 800-816.
- Culver M, Dworkin HJ (1991) Radiation safety consideration for postiodine- 131 hyperthyroid therapy. J Nucl Med 32(1): 169-173.
- Svensson J, Berg G, Wängberg B, Larsson M, Forssell-Aronsson E, et al. (2015) Renal function affects absorbed dose to the kidneys and hematological toxicity during 177Lu-DOTATATE treatment. Eur J Nucl Med Mol Imaging 42(6): 947-955.
- Garkavij M, Nickel M, Sjögreen-Gleisner K, Ljungberg M, Ohlsson T, et al. (2010) 177Lu-[DOTA0,Tyr3] Octreotate therapy in patients with disseminated neuroendocrine tumors: analysis of dosimetry with impact on future therapeutic strategy. Cancer 116(4 Suppl): 1084-1092.
- Sandström M, Garske U, Granberg D, Sundin A, Lundqvist H (2010) Individualized dosimetry in patients undergoing therapy with 177Lu- DOTA-D-Phe(1)-Tyr(3)-octreotate. Eur J Nucl Med Mol Imaging 37(2): 212-225.
- Garske U, Sandström M, Johansson S, Sundin A, Granberg D, et al. (2012) Minor changes in effective half-life during fractionated 177Lu-Octreotate therapy. Acta Oncol 51(1): 86-96.
- Larsson M, Bernhardt P, Svensson JB, Wangberg B, Ahlman H, et al. (2012) Estimation of absorbed dose to the kidneys in patients after treatment with 177Lu-octreotate: comparison between methods based on planar scintigraphy. EJNMMI Res 2(1): 49.
- Baechler S, Hobbs RF, Boubaker A, Buchegger F, He B, et al. (2012) Three-dimensional radiobiological dosimetry of kidneys for treatment planning in peptide receptor radionuclide therapy. Med Phys 39(10): 6118-6128.
- Esser JP, Krenning EP, Teunissen JJ, Kooij PP, van Gameren AL, et al. (2006) Comparison of [177Lu-DOTA0, Tyr3] octreotate and [177Lu- DOTA0, Tyr3]octreotide: which peptide is preferable for PRRT? Eur J Nucl Med Mol Imaging 33(11): 1346-1351.
- Richard W, Janine W, Kimberly S, Jimmy L, Jaime (Jim) S, et al. (2015) Radiation safety restrictions in the radionuclide treatment of canine osteosarcoma, J Nucl Med 56(suppl 3): 1226.
- Siegel JA, Thomas SR, Stubbs JB, Stabin MG, Hays MT, et al. (1999) MIRD pamphlet no. 16: techniques for quantitative radiopharmaceutical biodistribution data acquisition and analysis for use in human radiation dose estimates. J Nucl Med 40(2): 37S-61S.
- Heikkonen J, Mäenpää H, Hippeläinen E, Reijonen V, Tenhunen M (2016) Effect of calculation method on kidney dosimetry in Lu-177-octreotate treatment. Acta Oncol 55(9-10): 1069-1076.
- Miederer M, Reber H, Helisch A, Fottner C, Weber M, et al. (2012) One single-time-point kidney uptake from OctreoScan correlates with number of desintegrations measured over 72 hours and calculated for the 6.7 hours halflife nuclide 177Lu. Clin Nucl Med 37(10): e245-e248.
- Fernandez R, Sarah A, Val L, et al. (2015) Ensuring Safe & Effective Delivery of Lutetium-177 Dotatate Therapy. J Nucl Med 56(supplement 3): 479.
- J Archer, M Carroll, S Vinjamuri (2017) Clearance of 177Lu-DOTATATE from patients receiving peptide receptor radionuclide therapy Experiences at the Royal Liverpool University Hospital. RAD Magazine 39(455): 13-15.
© 2018 Mushref Algarni. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






