- Submissions

Full Text
Novel Approaches in Cancer Study
Advances of Radiation Oncology in Cancer Management: Vision for Role of Theranostics, Present and Future
Carlos A Perez*
Department of Radiation Oncology, Washington University, USA
*Corresponding author: Carlos A. Perez, M.D.1, Sasa Mutic, Ph.D.1 1Department of Radiation Oncology Mallinckrodt Institute of Radiology/ Siteman Cancer Center Washington University School of Medicine 660 South Euclid Ave, St Louis, MO, 63110 USA
Submission: September 22, 2017; Published: November 13, 2017

ISSN:2637-773XVolume1 Issue1
Abstract
Significant computational and technological advances in radiation therapy have enhanced our ability to more accurately plan and deliver increasing doses of radiation therapy to limited target volumes in many patients with cancer. Recent developments on magnetic resonance on-line imaging and use of implanted markers allow more precise on-time tumor localization with lower doses delivered to surrounding organs at risk leading to less treatment morbidity.
Biological markers and molecular imaging (theranostics) will add new dimensions and precision to radiation therapy techniques. Nanoparticles are promising tools in therapeutic programs. Further research in efficacy, safety, cost utility (value) and institution of robust quality assurance programs will be necessary to optimize these contributions in clinical practice.
Keywords: Radiation Oncology; Advances; Future; Molecular Imaging; Nanotechnology; Theranostics
Abbreviations: CT: Computed Tomography; IMRT: Intensity Modulated Radiation Therapy; PET: Positron Emission Tomography; GMI: Global Mutual Information; BEP: Bending Energy Penalty; NCC: Normalized Cross Correlation; IGRT: Image-Guided Radiation Therapy; OAR: Organs At Risk; FDG: Fluorodeoxyglucose; FLT: 18F Labeled Thymidine VEGF: Vascular Endothelial Growth Factor; HIF-1 hypoxia-inducible factor 1; GLUT1: Glucose Transporter; MCT4: Monocarboxylate Transporter DCE: Dynamic Contrast Enhanced; ER: Estrogen Receptor; CNS: Central Nervous System; GNP: Gold Nanoparticles; GBS: Gold-loaded Brachytherapy Spacer; QIN: Quantitative Imaging Network; QI: Quality Improvement; QIBA: Quantitative Imaging Biomarkers Alliance; EuroCAT: Euregional Computer Assisted Theragnostics project
Advances in Radiation Oncology
Radiation Oncology is one of the pillars of multidisciplinary care of the patient with cancer, along with surgery, chemotherapy, targeted therapy and immunotherapy. Since the introduction of computed tomography (CT) scanning in the early 1970's and three-dimensional conformal radiation therapy (3D-CRT) in 1985, Radiation Oncology has experienced dramatic technological innovations, leading to image-based three-dimensional treatment planning and delivery of radiation therapy, using 3D-CRT, Intensity Modulated Radiation therapy (IMRT), stereotactic techniques (SRS and SBRT) with increased precision in dose delivered to the target volume(s), while sparing adjacent normal structures (Organs at Risk, OAR) [1,2]. The dose-rate effect of external beam radiation therapy with conventional linear accelerators is governed by the overall beam-on-time.
At the same time, exponential growth of medical imaging modalities (CT, MRI, ultrasound), including image fusion have enhanced our ability to diagnose cancer at earlier stages and to more accurately stage the tumor. The improvement in staging accuracy has enabled us to better tailor therapy based on the characteristics of the individual patient and tumor features and extent.
PET and Molecular Imaging
In the past 10 years there has been an increasing use of functional PET imaging (18F-FDG and other specific radiotracers for hypoxia, cellular proliferation, angiogenesis, etc) or magnetic resonance spectrometry, provide better delineation of target volumes, particularly clinical target volumes [3]. Radiomics, is defined as a noninvasive imaging method of assessing the tumor and its microenvironment in their entirety, which allows the evaluation and monitoring of tumor characteristics such as temporal and spatial heterogeneity. The process of radiomics consists of discrete steps: image acquisition and segmentation, feature extraction, statistical learning and 3D rendering. Nevertheless, as noted in a review by Limkin et al. [4] the use of radiomics as clinical biomarkers still necessitates standardization in order to achieve routine implementation in clinical practice.
Combined positron emission tomography (PET)/magnetic resonance imaging (MRI) is highly promising for biologically individualized radiation therapy (RT). Leibfarth et al. [5] reported on an exploratory study of 8 patients consisting of an FDG PET/ (CT) and a subsequently acquired PET/MR of the head and neck (HN). Following a rigid registration, deformable registration with a transform parametrized by B-splines three different optimization metrics was investigated: global mutual information (GMI), GMI combined with a bending energy penalty (BEP) for regularization (GMI1 BEP) and localized mutual information with BEP (LMI1 BEP). Different quantitative registration quality measures were developed for structures segmented on CT and MR as well as anatomical landmark distances. Moreover, the local registration quality in the tumor region was assessed by the normalized cross correlation (NCC) of the two PET datasets. LMI1 BEP yielded the most robust and accurate registration results.
The authors concluded that accurate and robust multimodal deformable image registration of CT and MR in the HN region could be performed using a B-spline parametrized transform and LMI1 BEP as optimization metric. With this strategy, biologically individualized RT treatment planning based on combined PET/MRI to achieve volumetric dose optimization (defined as dose painting) is possible.
Treatment Planning/Delivery and On-line Imaging Devices
With the advent of robust three-dimensional treatment planning systems, technologically refined flattening-filter-free linear accelerators and hypofractionation (high dose per fraction, lower total dose) radiation therapy schemas, biological effects of external beam dose rate will need further investigation. Requirements on dose conformality, smaller PTV margins and the sharp peripheral dose gradient of new treatment devices require a more stringent daily tumor volume localization techniques and Quality Assurance Program. Widespread use of electronic clinical and dosimetry records and informatics methodology will add reliability to the data acquired.
In-room on-line imaging devices, including Cone Beam CT (CBCT), commercially available have facilitated the implementation of Image-Guided Radiation Therapy (IGRT). The recent introduction of MRI imaging in real time, robust treatment planning algorithms and delivery of radiation therapy, initially with 60Co sources and later with a linear accelerator has optimized the accuracy in localization of the target volume during exposure to therapeutic radiation (View Ray in Mountain View, CA). Heavy particles and protons, already in clinical use in many countries will continue to expand. It will be critical to establish the potential cost benefit of these modalities and identify selected group of patients to be treated with them [6,7]. Implanted target tracking devices enhance our ability to more accurately localize the target for treatment. 4D CT scanning with faster scanners and treatment planning has facilitated correction of target motion in lung and upper abdomen, including Active Breathing Control Techniques.
As a corollary, stereotactic hypofractionated techniques have been introduced in the treatment of brain, lung, and tumors in other anatomical sites. While the on-line Cone Beam CT and other technologies are more refined, we are still unable to image and quantify the true volume of soft tissue tumors on daily basis during radiation delivery [8]. This undoubtedly remains one of the frontiers in radiation oncology and the recent technological developments indicate that we will have increasing capabilities in the upcoming years. Vernekohl et al. [9] showed that the combination of XF CT and Compton scatter imaging is a valid path to realize molecular imaging with high atomic number probes for radiation therapy. Existing on-line Imaging systems can be upgraded with advanced detector technology to enable molecular imaging, given the constraints of energy resolution and limited radiation dose exposure, spatial resolutions of some millimiters and molecular sensitivities.
Internal implantable electromagnetic or transmission dosimeters may eventually facilitate the verification of actual radiation dose administered to the target and organs at risk (OAR).
Potential for Theranostics in the Future of Radiation Therapy
Molecular imaging provides unique information on the tumor phenotype (tumor cells and tumor microenvironment), influencing treatment decisions and adaptation of therapy by early prediction of treatment outcome. With the increasing importance of systemic targeted therapies and of high precision external beam radiation therapy, identifying tumor characteristics before and during therapy becomes increasingly important. Key features like tumor metabolism, microenvironment and hypoxia, cellular proliferation rate and protein synthesis can be depicted by radiopharmaceuticals such as fluorodeoxyglucose (FDG), 18F labeled thymidine (FLT), hypoxia markers, etc.
For instance, Vera et al. [10] investigated the changes in tumor proliferation (using FLT), metabolism (using FDG), and hypoxia (using F-misonidazole) during curative chemo- radiotherapy (CT-RT) in 30 patients with non-small-cell lung cancer (NSCLC). Candidates for curative-intent CT-RT. Three PET-CT (Biograph S16, Siemens) scans were performed before (t0) and during (around dose 46 Gy, t46) RT with minimal intervals of 48 h between each PET-CT scan. The tracers used were FDG for metabolism, FLT for proliferation, and 18F-misonidasole (F-miso) for hypoxia. The 3 image sets obtained at each time point were co-registered (rigid: n = 9, elastic: n = 1, Leonardo, TrueD, Siemens) using FDG PET-CT as reference. Volumes of Interest (VOI) were delineated (40% SUVmax values used as a threshold) for tumors and lymph nodes on FDG PET-CT, and they were automatically pasted on FLT and F-miso PET-CT images. ANOVA and correlation analyses were used for comparison of SUVmax values.
Four tumors and twelve nodes were identified on initial FDG PET-CT images. FLT SUVmax values were significantly lower (p < 0.0006) in both tumors and nodes. The decrease in FDG SUVmax values had a trend towards significance (p = 0.048). F-Miso SUVmax values were significantly higher in tumors than in lymph nodes (p = 0.02) and did not change during radiation therapy (p = 0.39). A significant correlation was observed between FLT and FDG uptake (r = 0.56, p < 10_4) when all data were pooled together, and they remained similar when the before and during RT data were analyzed separately. FDG and F-miso uptakes were significantly correlated (r = 0.59, p = 0.0004) when all data were analyzed together.
Furthermore, imaging receptor expression on tumor cells, for example with radiolabeled antibodies and peptides, can provide information on the presence, heterogeneity, accessibility, and modulation of these receptors for targeted therapies. Small molecules like Tyrosine Kinase Inhibitors radiolabeled may provide insight in tumor pathophysiology allowing better selection of patients likely to respond to a specific treatment. A distinct form of theranostics utilizes targeting molecules, radiolabeled with gamma or positron emission for imaging, selecting patients for treatment and may allow monitoring of response to therapy [11]. Classical radio- and chemo-therapy resistance mechanisms include DNA-repair capacity, tumor repopulation and hypoxia, for which various biomarkers have been identified. For a biomarker assay to be successful for wide clinical application it should preferably be non-invasive, fast, not too complex, and suited for repetitive assessments. PET-scanning meets these criteria although specific tracer availability can be a limitation [12].
Hypoxia Biomarkers and Molecular Imaging
Radiation therapy tumor cell kill relies on induction of oxidative stress. The response of malignant tumors to irradiation varies as a consequence of resistance mechanisms at the molecular level. Thorwarth et al. [13] published a detailed description of methodological aspects of PET image acquisition and processing to assess tumor hypoxia. During fractionated radiation therapy, HIF1alpha protects the tumor microvasculature from radiation- induced endothelial apoptosis, via induction of vascular endothelial growth factor (VEGF) and other pro-angiogenic factors and facilitates tumor cell survival by increasing the antioxidant capacity of tumors to counteract radiation-induced oxidative stress [14]. Irradiation also induces changes in the tumor microenvironment such as vascular, stromal, and immunological changes which may promote radioresistance and tumor recurrence. These effects eventually lead to the resistance of tumor cells to chemotherapy and radiation [15].
The hypoxia-inducible factor 1 (HIF-1) pathway is involved in several of these processes. With respect to metabolism, HIF-1 is an important regulator of glycolysis and the pentose phosphate pathway. This aberrant cellular metabolism, responsible for maintaining stable intracellular ATP levels without oxygen consumption even under normoxic conditions, increases the antioxidant capacity of tumors, thereby countering the oxidative stress caused by irradiation. Analysis of glucose transporter (GLUT1) and monocarboxylate transporter (MCT4) expression on the histological level suggested a different metabolism for adenocarcinomas and squamous cell carcinomas of the lung.
Results showed that adenocarcinomas rely mainly on aerobic glycolysis, whereas the energy metabolism of squamous cell carcinomas is more physiologically, i.e. mitochondrial oxidation with glycolysis only under hypoxic conditions. This indicates that adenocarcinomas exhibit glycolysis under normoxic conditions, whereas squamous cell carcinomas are exposed to diffusion- limited hypoxia resulting in a very high anaerobic glycolytic rate. Consequently, the FDG PET interpretation based on histology improves its prognostic and predictive potential prior to treatment and allows monitoring of treatment efficacy during treatment [16]. Scientists at the University of Cambridge created a technique with an imaging device using a combination of light and sound to check the oxygen levels in prostate tumors in mice; the researchers imaged the stronger response to oxygen by tumors with better blood vessels. This could help to find patients with more difficult to treat prostate cancers as cancer cells supplied by poor quality blood vessels and low oxygen levels (hypoxia) are more resistant to drugs and radiation therapy. Also, cells in hypoxic conditions are better at adapting to harsh conditions and make them biologically more aggressive. Poor blood vessels also reduce the number of treatments like hormones or chemotherapy that can penetrate into the center of the tumor. If we could translate this technology to the clinic, it could provide a noninvasive way to stratify men for treatment and monitor the response.
18F-Fluciclovine (anti-1-amino-3-[18F] fluorocyclobutane-1- carboxylic acid) is a novel PET)-CT radiotracer that has demonstrated utility for detection of prostate cancer. Schreibmann [17] reported initial findings from a cohort of 41 patients, in a randomized controlled trial; patients were randomized to the 18F-fluciclovine PET-CT for the detection of metabolic abnormalities and highresolution CT for treatment planning. In 21 of 55 abnormalities, a deformable registration was needed to map the 18F-fluciclovine activity into the simulation CT. The most selected percentage was 50% of maximum SUV, although values ranging from 15% to 70% were used for specific patients. The inclusion of 18F-fluciclovine changed the planning volumes for 46 abnormalities (83%) of the total 55, with 28 (51%) located in the lymph nodes, 11 (20%) in the prostate bed, 10 (18%) in the prostate, and 6 (11%) in the seminal vesicles. Only 9 PET abnormalities were fully contained in the standard target volumes based on the CT segmentations and did not necessitate expansion. The authors concluded that use of 18F-fluciclovine in postprostatectomy radiation therapy planning led to augmentation of the target volumes in 30 of the 41 patients studied.
Bentzen, Gregoire [18] reviewed the potential application of PET tracers: such as FDG and choline as surrogates for tumor burden, fluorothymidine to image proliferation (or cellular growth fraction) and hypoxia sensitive radionuclides, including 18Ffluoromisonidazole, EF3, EF5, and 64Cu-labeled copper (II) diacetyl-di (N4-methylthiosemicarbazone) as surrogates of cellular hypoxia. They discussed research advances supporting the clinicobiological rationale for dose painting (in our opinion a misnore, as volumetric biological dose optimization is more appropriate designation) and they reviewed aspects of the technical feasibility of optimizing and delivering realistic optimized doses of radiation therapy to specific tumor sub-volumes.
One potential future application of molecular imaging in radiation therapy planning is in the biological delineation of tumor sub volumes for dose escalation using biologically guided volumetric treatment planning and dose optimization. The rationale for biologically guided radiation dose optimization comes from the fact that tumors are biologically heterogeneous in composition and often show nonuniform patterns of response to a generally accepted therapeutic dose of radiation therapy. Molecular imaging may be able to identify spatial patterns of radioresistance, which can be used to guide and shape the administration of additional radiation dose to limited volumes, improving tolerance to higher doses. Indirect evidence of this principle comes from studies that have found molecular imaging biomarkers to correlate with patient outcome after radiation therapy [19,20]. Possible biologic targets include tumor hypoxia (measured with PET hypoxia radiotracers or with dynamic contrast-enhanced [DCE] MRI), cellularity (measured with 18F-FDG PET or DW MRI), and others. Identifying effective and robust imaging-based targets for additional volumetric planned radiation therapy is currently an ongoing research activity [21]. Volumetric dose optimization has yet to be validated as an effective treatment option, but preliminary clinical studies have evaluated its efficacy and acceptable treatment morbidity. Studies have been performed on the brain using 18F-FDG and O-(2-18F-fluoroethyl)-L- tyrosine PET [22,23], on head-and-neck tumors using 18F-FDG PET [24-26], on prostate tumors using 18F-fluorocholine PET [27], and on lung tumors using 18F-FDG PET [28].
The potential for using molecular imaging to discriminate radiosensitive tissue from radioresistant tissue in tumors may be equally valuable when applied to the surrounding normal tissue [29,30]. Certain regions in an organ, such as the lung, appear to be more sensitive to radiation therapy than other regions in the same organ. Or some regions may be more functional than others and therefore more important to spare. For example, in 18F-FDG PET imaging of the lungs, studies have found that regions of uptake before radiation therapy are more likely to experience radiation therapy toxicity [31]. Ventilation imaging, acquired with a variety of methods, may identity lung regions that are blocked-either by obstructive lung disease or by tumor burden-and may contribute less to the overall lung function than healthy, functioning lung.
For MRI, hyperpolarized gases or gadolinium aerosol can be used in combination with serial imaging to produce 4D images of lung ventilation [32]. Ventilation maps can also be derived from 4D CT imaging, in which voxel Hounsfield units correlate with the fraction of air in the voxel volume [33]. In SPECT, 99mTc- labeled macro aggregated albumin can also be used to create 3D ventilation maps. The feasibility of incorporating lung ventilation maps into treatment planning for conformal avoidance has been demonstrated [34]. Although promising, the clinical benefit of using functional images for conformal avoidance has yet to be validated. Considering the extensive ongoing clinical research and continuous technologic advances; we envision that the methods presently considered to be advanced will soon become routine practice. For a complete review on molecular imaging in radiation therapy the reader is directed to an excellent publication, including imaging techniques by Jeraj et al. [29].
Lei et al. [35] and Cheng et al. [36] investigated whether luoropropane 18F-FMISO]) PET/CT could predict primary resistance to hormonal therapy in ER-positive breast cancer. Postmenopausal women who had ER-α-positive breast cancer, stages II-IV, and had never received prior endocrine therapy were prospectively enrolled in this study. Patients underwent both 18F-FDG and 18F-FMISO PET/ CT scans before and after treatment. 45 lesions (13 primaries, 32 metastatic) from 20 patients met the inclusion criteria in this study. Baseline (18) F-FDG and (18) F-FMISO PET/CT scans were obtained for 33 lesions from 16 patients. The correlation between baseline 18F-FDG uptake and clinical outcome was weak and did not reach statistical significance (r = 0.37, P = 0.031). However, there was a significantly positive correlation between baseline 18F-FMISO uptake and clinical outcomes after ≥3 mo of primary endocrine therapy with letrozole (< 0.0001).
PET Imaging and Estrogens in Breast Cancer
The estrogen derivative 16α-F-fluoro-17β-estradiol (FES) is a PET tracer that has been used in a variety of preclinical and clinical studies to detect estrogen receptor (ER) expression, mainly in breast cancer, but also for other oncological indications [37].
Yoon et al. [38] reported on 43 patients with large or locally advanced invasive ductal carcinoma. (68) Ga-Labeled arginine- glycine-aspartic acid (RGD) and (18) F-fluorodeoxyglucose (FDG) positron emission tomography/computed tomography (PET/CT). Quantitative FDG PET parameters were significantly higher in the ER-negative group (15.88 vs 10.48 p = 0.02). Only the ER/PR-, Her2- subgroup showed a significant positive correlation between FDG and RGD PET parameters (r = 0.59, p = 0.03 for SUVmax). Furthermore, Linden and Deshdashti [39] reviewed novel tracers in breast cancer including steroidal endocrine tracers, 16α-18F] fluoro-17β-estradiol (FES) to measure tumor estrogen receptor density and function and 21-(18)F-fluoro-16α,17α-[(R)-(1'-α- furylmethylidene)dioxy]-19-norpregn-4-ene-3,20-dione (FFNP) to assay tumor progesterone receptor (PgR) expression, and to asses nuclear proliferation using 3’-deoxy-3’-fluorothymidine (FLT), membrane lipids using (11)C- or (18)F-labeled choline and amino acid transport using (11)C-methionine. They felt these investigational tracers were moving closer to clinical use, and were likely to affect patient care by aiding in characterization of breast cancer biology, which could have an important effect in the selection of targeted therapy and monitoring response to such therapy.
16α-(18) F-fluoro-17β-estradiol ((18) F-FES) is an estrogen receptor (ER)-specific PET tracer with various potential applications. In a study of thirty-three patients who underwent 18F-FES PET to evaluate equivocal lesions on conventional workup (n = 21), ER status in metastatic patients (n = 10), and the origin of metastases (n = 2). 18F-FES-positive lesions were observed in 22 patients. 18F-FES PET was especially sensitive for bone metastases, detecting 341 bone lesions, compared with 246 by conventional imaging. The sensitivity for liver metastases was poor, and quantification of 18F-FES uptake in liver lesions was hampered by high physiologic background. (18)F-FES uptake was highly variable between all metastases (range of standardized uptake value, 1.2018.81), and 45% of the patients with a positive (18) F-FES PET finding had both (18) F-FES-positive and (18) F-FES-negative metastases. (18) F-FES PET improved diagnostic understanding in 88% of the patients and led to therapy change in 48% of the patients [40]. Medical imaging in addition to CT scanning, MRI (with contrast agents), ultrasound, includes fluorescent markers (organic dyes and inorganic quantum dots), or in vitro lab test [41] including DNA sequencing [42] and often involve deep learning algorithms that weigh the result of testing for several biomarkers [43].
Potential Role of Nanoparticles
A document by an ASTRO Task Force on recommendations for research in biological sciences of radiation oncology included the identification of imaging targets of relevance to radiation treatment and evaluation of radiation response, the development of specific molecular imaging probes and modalities, the incorporation of these methods into the clinical radiation therapy workflow, and the critical evaluation of the benefits of these novel technologies for patients [44].
An area with potential application in clinical radiation oncology is the use of nanoparticles with specific target radiotracers for imaging or radiopharmaceuticals for therapeutic purposes [45]. Dual modality X-ray/optical cancer imaging allows for detailed molecular information to be co-registered with anatomical CT images. The development of brightly emitting X-ray radioluminescent probes is crucial for effectively utilizing the combined anatomical X-ray and molecular optical imaging techniques to visualize tumors with molecular and cellular precision. Currently, the detection of visible light from X-ray radioluminescent materials limits both imaging depth and resolution due to attenuation and scattering of the emitted photons [45]. Naczynzki and colleagues [46] showed in mice bearing tumor xenografts that SWIR or NIR-II light (10002300 nm) holds significant promise for molecular imaging and therapeutic assessment in radiobiological studies, by avoiding the considerable absorbance and scattering observed by visible light as it passes through living tissue. Both kV and MV photons were used to examine the photon yields of the phosphor nanoparticles. SWIR imaging showed X-ray radioluminescence can be used to identify the presence of nanoparticles in tumor tissue and organs responsible for nanoparticle clearance. Tissue phantom studies confirmed the improvements in imaging resolution and depth afforded to SWIR compared to visible X-rays.
In a review of technological innovations in radiation oncology published by an ASTRO panel [47] it was noted that despite the promise afforded by nanoparticle systems, challenges exist: toxicity is a concern, and nanoparticles (even gold-based systems) will need to be extensively tested for safety and biocompatibility before being used in human trials; stability in size and form of nanoparticles or their delivery vehicles if particles lose their form or cluster together in circulation and are opsonized by plasma proteins, their delivery to tumors and targeting efficiency may be significantly dampened; potency of the amount of agent taken up in the target to observe an improvement in therapy needs to be validated; the clinical feasibility may be severely limited except for those applications that do not require tissue penetration (e.g. targeting tumor vasculature); targeting specificity whereas passive targeting, relying on the intrinsic enhanced permeability and retention properties of tumors is an effective method for preferential nanoparticle accumulation in the tumor, active targeting through ligands, peptides, or other methods has been shown to provide greater specificity for some situations; and feasibility, clinical workflow and costs, among other factors, will need to be addressed.
Hassanzaden et al. [48] pointed out the limited efficiency of the current treatment options against central nervous system (CNS) disorders and the research efforts in nanotechnology that have led to the production of highly-advanced nanodevices and biomaterials in a variety of geometries and configurations for targeted delivery of genes, drugs, or growth factors across the blood-brain barrier. They noted that computational modelling has emerged as a powerful tool for rational design of nanoparticles with optimized characteristics including the selectivity, improved bioactivity, and reduced toxicity that might lead to the effective delivery of therapeutic agents. High-performance simulation techniques by shedding more light on the dynamical behavior of neural networks and pathological mechanisms of CNS disorders may provide imminent breakthroughs in nanomedicine. The authors reviewed the importance of integrating nanotechnology-based approaches with computational techniques for targeted delivery of theranostics to the CNS. Obviously, this technology could be potentially combined with radiation therapy to enhance anti-tumor activity.
Brachytherapy, implanting radioactive sources in a tumor is an application par excellence of Adaptive Radiation Therapy, limiting the radiation dose to the target volume and a restricted margin affecting normal tissues. These characteristics have been amplified with the use of magnetic resonance imaging to more accurately delineate volumes of interest [49]. Shihan et al. [50] noted that some recent studies concluded that administering gold nanoparticles (GNP) to cancer cells during brachytherapy could lead to significant dose enhancement to a tumor. However, delivery of sufficiently potent concentrations of nanoparticles into solid tumors remains a challenge, mostly attributed to the physiological barriers imposed by the abnormal tumor vasculature and the dense interstitial matrix, a complex assembly of collagen, glycosaminoglycan, and proteoglycans, which may hinder deep penetration of the nanoparticles. To overcome this challenge they proposed a biological in situ dose optimization approach in which inert brachytherapy spacers, routinely used for increasing spatial accuracy during brachytherapy, could be loaded with radiation- sensitizing drugs in gold nanoparticles (GNP) to be released or eluted in situ after implantation, to enhance therapeutic ratio.
They explored the feasibility of loading the inert brachytherapy spacers with drugs. The sustained release of GNP in situ from the gold-loaded brachytherapy spacer (GBS) and consequent 3-dimensional intratumor biodistribution over time could then be customized by varying GNP size, initial concentration, and other factors to enhance brachytherapy effect in desired tumor subvolumes. Because implantation of inert spacers is already part of routine clinical practice, replacing the inert spacers with GBS would come at no additional inconvenience to patients. The theoretical feasibility of this potential new approach was explored by investigating the intratumor biodistribution and corresponding dose enhancement over time for GNP released from the GBS as a function of nanoparticle size for the different low dose-rate (LDR) brachytherapy sources iodine-125 (I-125), palladium-103 (Pd- 103), and cesium-131 (Cs-131).
Guo et al [51] discussed the development of multifunctional nanomaterials. Gold nanoparticles (AuNPs) now being widely utilized in bio-imaging and phototherapy due to their tunable and highly sensitive optical and electronic properties (the surface plasmon resonance). That may have diagnostic and therapeutic functions. They reviewed important properties of AuNPs relevant to diagnostic and phototherapeutic applications such as structure, shape, optics, and surface chemistry and discussed barriers for translational development of theranostic AuNPs and recent advances in the application of AuNPs for cancer diagnosis, photothermal, and photodynamic therapy.
As noted by Pradeep et al. [52] future research in the nanoparticle area should focus on clinically relevant bioactive combinations, better metastasis control, integration of imaging and theranostic techniques, predictive animal/pre-clinical models, maximal utilization of extra- and intracellular tumor microenvironment for drug delivery, and exploring the metabolomic-, proteomic-, and genomic-based personalization of cancer nanomedicine.
Precision Medicine
Precision medicine is an approach for disease treatment and prevention that takes into account individual variability in genes, environment, and lifestyle; integrating information from multiple sources with the end goal of personalized management. In personalized medicine, diagnostic procedures and prognostic/ predictive markers often select appropriate subsegments of patients for more specific optimal therapies based on the patient's genetic profile or other molecular or cellular analysis. The use of genetic information has played a major role in certain aspects of personalized medicine (e.g. pharmacogenomics), and the term was first coined in the context of genetics, though it has since broadened to encompass all sorts of personalized medicine.
As noted by Kaanders et al. [53], integration of molecular imaging techniques into therapy selection and radiation treatment planning can serve several purposes. First, pretreatment assessments can steer decisions about radiation therapy modifications or combinations with other modalities. Second, biology-based objective functions can be introduced to the radiation treatment planning process by co-registration of functional imaging with planning CT-, MRI- or PET-scans, thus, optimized volumetric radiation dose distributions can be generated with escalating doses to macroscopic tumor (GTV), where radiation therapy resistance is most prevalent. Third, monitoring of temporal and spatial variations in radiation therapy resistance mechanisms early during the treatment can discriminate responders from non-responders. With such information available shortly after the start of the treatment, modifications can be implemented or the radiation treatment plan can be adapted according to the biological response pattern. Currently, these strategies are in various phases of clinical testing, mostly in single-center studies but more and more also in multi-center set-up. Ultimately, this should result in availability for routine clinical practice requiring stable production and accessibility of tracers, reproducibility and standardization of imaging and analysis methods and general availability of knowledge and expertise. Small studies employing adaptive radiotherapy based on functional dynamics and early response mechanisms demonstrate promising results. This approach is close to large scale clinical testing with good prospects of success.
Quality Assurance
Regardless of the radiation modality we must strive to optimize dosimetric precision in radiation treatment planning, delivery and verification. One of the key elements in delivery of radiation therapy that is still to be attained is accurate assessment of the true physical dose delivered to the target volumes in the patient (8). Medical imaging, in the hopefully not-too-distant future including molecular and nanotechnology will continue its vertiginous progress with additional refinements, molecular approaches and more specific radiotracers or radiopharmaceuticals that are being developed to identify distinctive tumor and microenvironment characteristics. The incorporation of molecular imaging, predictive biomarkers, nanotechnology and other theranostics advances will be challenging and will require careful planning and evaluation of their efficacy and safety [54].
Whereas radiation dose escalation is attractive and in some sites it has been shown to improve tumor control (i.e. prostate cancer), the new technological developments are particularly attuned to the use of hypofractionated, including stereotactic irradiation, which has not only biological rationale but ideally an impact on resource utilization and the cost of patient care and convenience. The above advances in tumor volume definition, computational capability and electronic engineering will amplify the opportunities to administer Adaptive Radiation Therapy on a real-time basis, to daily optimize dose, to accommodate anatomical deformation or changes in the volume, configuration or biological tumor characteristics using automated segmentation and to correct random or systematic positioning uncertainties.
Kong et al. [55] demonstrated that tumors significantly decrease in size and metabolic activity after delivery of 45 Gy of fractionated radiation therapy (RT), and that metabolic shrinkage is greater than anatomic regression. They carried out a phase II clinical trial at two institutions with 42 patients who had inoperable or unresectable stage II to stage III NSCLC to determine whether 18F- with fludeoxyglucose-positron emission tomography/computed tomography (FDG-PET/CT) acquired during the course of treatment provided an opportunity to deliver higher-dose radiation to the more aggressive areas of the tumor to improve local tumor control without increasing RT-induced lung toxicity (RILT), and possibly improve survival. Conformal RT was individualized and adaptively escalated to the residual tumor defined on mid treatment FDG-PET up to a total dose of 86 Gy in 30 daily fractions. Medically fit patients received concurrent weekly carboplatin plus paclitaxel followed by 3 cycles of consolidation.
Median tumor dose delivered was 83 Gy (range, 63-86 Gy) in 30 daily fractions. Median follow-up for surviving patients was 47 months. The 2-year rates of infield and overall local regional tumor controls (i.e., including isolated nodal failure) were 82% and 62%, respectively. Median overall survival was 25 months (95%CI, 1232 months). The 2-year and 5-year overall survival rates were 52% and 30%, respectively.
We envision further refinements in designing more versatile and reliable software, remote computational techniques and automation for treatment planning and delivery, to enhance efficiency and safety in the processes of radiation therapy. Further, the voluminous amount of clinical and dosimetry data generated with contemporary techniques requires a robust processing system, which in the future will be strengthened with refined informatics and use of cloud computing for data acquisition, analysis and storage.
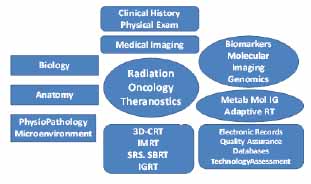
The advances in speed and scope of imaging technologies used in radiation therapy, will allow us to quantify anatomical and biological characteristics of patient anatomy at an increased frequency and some aspects of this is already used for daily patient assessments and the scope of these assessments will continue. It is also likely that there will be a continuation in increase of image acquisition during the actual treatment delivery. These evolutions in imaging capabilities coupled with fast contour delineation and fast dose computation should allow us to more adequately quantify true doses delivered to target volumes and normal structures and to assess their response to treatment. The increased availability of such technology along with cloud based infrastructure should also accelerate the implementation of these processes for a large number of patients. Potential application in Brachytherapy is the use of nanoparticles with specific target radiotracers for imaging or radiopharmaceuticals for therapeutic purposes [56]. Figure 1 depicts the integrated structure of a Radiation Oncology Program incorporating Theranostics.
Figure 1: Schematic representation of Integrated Radiation Oncology Structure, including Theranostics.
3D-CRT= Three Dimensional Conformal Radiation Therapy
IMRT= Intensity Modulated Radiation Therapy
SRS= Stereotactic RadioSurgery
SBRT= Stereotactic Body Radiation Therapy
IGRT= Image guided Radiation Therapy
Metab Mol Adaptive RT= Metabolic Molecular Adaptive Radiation Therapy
As pointed out by Scheinberg
As pointed out by Scheinberg et al. [57] the use of nano-scale size materials in devices, drugs and diagnostic agents comes with a number of new opportunities, and also serious challenges to human applications. The larger size of particulate-based agents, as compared to traditional drugs, allows for significant advantages of multivalency and multi-functionality. However, the human use of nanomaterials requires a thorough understanding of the biocompatibility of the synthetic molecules and their complex pharmacology. Possible toxicities created by the unusual properties of the nanoparticles are neither well-understood, nor predictable yet. A key to the successful use of the burgeoning field of nanomaterials as diagnostic and therapeutic agents will be to appropriately match the biophysical features of the particle to the disease system to be evaluated or treated.
Because of growing concern world-wide with the ever- increasing cost of health care and of sophisticated new technology we will need to strengthen our efforts in using cost benefit and comparative effectiveness techniques to document the gains that innovative technology may provide in patient care. Complex technology is associated with a potential increased risk on unintended undesirable effects. We must remain extremely vigilant and continuously establish and apply in our daily practice Quality Assurance Programs that will ensure the safety of our patients (and colleagues) and enhance the precision in planning and delivery of radiation therapy, as well as the reliability of data in clinical trials [58,59].
As emphasized by Limkin et al (4) improving multi-disciplinary network and dissemination of radiomics applications in oncology demand significant efforts. The National Cancer Institute, in cooperation with other societies like the Canadian Institute of Health Research, Cancer Research United Kingdom and American College of Radiology Imaging Network, have supported initiatives, including the Quantitative Imaging Network (QIN), to promote the development of Quality Improvement (QI) methods, annotated image databases, and QI standards. Other significant efforts include the Quantitative Imaging Biomarkers Alliance (QIBA), a critical component of US' Cancer Moonshot initiative, and the Euregional Computer Assisted Theragnostics project (EuroCAT), whose goals include enhancing data sharing and facilitating patient recruitment in clinical trials. More initiatives are necessary, with multidisciplinary working groups that include oncologists, radiologists, radiation oncologists, medical physicists, applied mathematicians, and computer scientists, to improve the field and educate people on its use such that it can become a reliable part of a decision support system in oncology.
As noted by Bentzen [60], even with a validated three dimensional (3D) volumetric imaging of the target, temporal stability (time function) is a concern. The usefulness of theranostic imaging in prescribing four-dimensional dose distributions obviously depends on the short-term and long-term stability of the 3D mapping of density of specific cellular phenotypes or microenvironmental variables. Oxygenation is one example: intermittent closing and opening of vessels can cause microscopic changes in oxygenation, so-called acute hypoxia, on a typical timescale of minutes [38,39]. The extent to which this process affects hypoxia as estimated with radionuclidelabelled compounds is not clear. In addition, there is reoxygenation of hypoxic regions after the radiation cell killing a few hours after each dose fraction and throughout the full 6-7 weeks of a fractionated course of radiation therapy. Even if tumor reoxygenation occurs, boosting of the radiation dose to the region that was hypoxic at the start of therapy can still be worthwhile and more important additional dose to residual biologically viable tumor subpopulations after standard radiation doses is critical to enhance local/ regional tumor control.
Spatial resolution is poorer with PET, SPECT, and MR spectroscopy than with MR imaging and CT scanning and there can be difficulties with partial volume artefacts. At present, commercial high-resolution clinical PET scanners have a full width at halfmaximum resolution of 3^5-5^0 mm in both the axial and transverse planes. The development of CT-PET and MR-PET scanners might help in reducing the partial volume artefacts and could improve the accuracy of image co registration. As stated in Bnetzen's review [59] by contrast, the achievable resolution with current PET scanners should be seen on the scale of achievable spatial precision in delivering 35 radiation fractions in the clinic, which will require an effective limit on the steepness of gradients that are deliverable with current radiation therapy technology, probably not better than 3-4 mm. Clearly, volumetric dose optimization will need optimum precision of the treatment planning dosimetry and the multileaf-collimator technology as well as patient immobilization and reproducibility. Equally critical to obtain the best result will be technology to allow real-time target localization during the radiation therapy exposure on the daily fraction administration. Physiological organ movement is another possible limitation that is being addressed by several research groups.
A challenging topic of research emphasized by Bentzen [60] is to establish the prescription function that is the mathematical link between a specific value of an imaging variable and the optimal clinical dose to be prescribed to the corresponding voxel. This function is unlikely to be defined from radiobiological measurements made in vitro but will have to be derived from outcome data in human beings or in animal experiments, evidence-based prescription rules that should be derived from controlled clinical trials. Simple linear interpolations between a clinically justified minimum and maximum dose over the range of values covered by the imaging variables might suffice as a first approximation, but strategies are under investigation that could further refine this prescription function.
Obviously, the above factors and considerations add significant complexities to the Quality Assurance programs that must be instituted as theranostics is incorporated in the clinical practice of radiation oncology.
Conclusion
Significant computational and technological advances in radiation therapy have enhanced our ability to more accurately plan and deliver increasing doses of radiation therapy to limited target volumes in many patients with cancer. Recent developments on magnetic resonance on-line imaging and use of implanted markers allow more precise real-time tumor localization with lower doses delivered to surrounding organs at risk, leading to less treatment morbidity. Biological markers and molecular imaging (theranostics) will add new dimensions and precision to radiation therapy techniques. Further research on systems design, efficacy, safety, cost benefit and institution of robust quality assurance programs will be necessary to optimize these contributions in clinical practice.
References
- Perez CA, Mutic S (2013) Advances and Future of Radiation Oncology. Rep Pract Oncol Radiother 18(6): 329-332.
- Mundt AJ, Roeske JC (2011) Image-Guided Radiation Therapy (IGRT): A Clinical Perspective: Peoples Medical Publishing House-USA.
- Ling CC, Gerweck LE, Zaider M, Yorke E (2010) Dose-rate effects in external beam radiotherapy redux. Radiother Oncol 95(3): 261-268.
- Limkin EJ, Sun R, Dercle L (2013) Promises and challenges for the implementationof computational medical imaging (radiomics) in oncology. Ann Oncol 28: 1191-1206.
- Leibfarth S, Monnich D, Welz S, Zips D, Thorwarth D, et al. (2013) A strategy for multimodal deformable image registration to integrate PET/ MR into radiotherapy treatment planning. Acta Oncol 52(7): 1353-1359.
- Balter JM, Haffty BG, Dunnick NR, Siegel EL (2011) Imaging opportunities in radiation oncology. Int J Radiat Oncol Bio Phys 79(2): 342-347.
- Levitt SH, Perez CA, Hui S, Purdy JA (2008) Evolution of computerized radiotherapy in radiation oncology: potential problems and solutions. Int J Radiat Oncol Bio Phys 70(4): 978-986.
- Jaffray DA, Lindsay PE, Brock KK, Deasy JO, Tome WA (2010) Accurate accumulation of dose for improved understanding of radiation effects in normal tissue. International journal of radiation oncol bio phys 76(3): S135-S139.
- Vernekohl D, Ahmad M, Chinn L (2016) On-Board Molecular Imaging (OBMI) for Radiation Therapy. Int J Radiat Oncol Bio Phys 96(2): S99.
- Vera P, Bohn P, Thiberville L, Dubray B, Edet-Sanson A, et al. (2011) Simultaneous positron emission tomography (PET) assessment of metabolism with 18F-fluoro-2-deoxy-D-glucose (FDG), proliferation with 18F-fluoro-thymidine (FLT), and hypoxia with 18fluoro- misonidazole (F-miso) before and during radiotherapy in patients with non-small-cell lung cancer (NSCLC): A pilot study. Redi other Oncol 98(1): 109-116.
- Oyen AJG (2014) Molecular imaging for theranostics. Radiother Oncol 118: S80.
- Kaanders JAHM, Hoeben BA, Bussink J (2015) Molecular imaging of proliferation and hypoxia. Radiother Oncol 115: S258.
- Thorwarth D, Minnich D, Zipps D (2013) Methodological aspects on hypoxia PET acquisition and imaging processing. Q J Nucl Med mol imaging 57(3): 235-243.
- Meijer TW, Kaanders JH, Span PN, Bussink J (2012) Targeting hypoxia, HIF-1, and tumor glucose metabolism to improve radiotherapy efficacy Clin Cancer Res 18(20): 5585-5594.
- Challapalli A, Carroll L, Aboagve EO (2017) Molecular mechanisms of hypoxia in cancer. Clin Transl Imaging 5(3): 225-253.
- Bussink J, Meijer TW, Kaanders JAHM (2015) Tumour metabolism is a critical factor for molecular imaging 115: S7-S8.
- Schreibmann E, Schuster DM, Rossi PJ, Cooper S, Jani AB, et al. (2016) Image Guided Planning for Prostate Carcinomas With Incorporation of Anti-3-[18F]FACBC (Fluciclovine) Positron Emission Tomography: Workflow and Initial Findings From a Randomized Trial. Int J Radiat Oncol Bio Phys 96(1): 206-213.
- Bentzen SM, Gregoire V (2011) Molecular Imaging-Based Dose Painting: A Novel Paradigm for Radiation Therapy Prescription. Semin Radiat Oncol 21(2): 101-110.
- Mortensen LS, Johansen J, Kallehauge J, Nordsmark M, Overgaard J, et al.(2012) FAZA PET/CT hypoxia imaging in patients with squamous cell carcinoma of the head and neck treated with radiotherapy: results from the DAHANCA 24 trial. Radiother Oncol 105(1): 14-20.
- Bradshaw TJ, Bowen SR, Deveau MA, Forrest LJ, Jeraj R, et al. (2015) Molecular imaging biomarkers of resistance to radiation therapy for spontaneous nasal tumors in canines. Int J Radiat Oncol Biol Phys 91: 787-795.
- Bowen SR, Chappell RJ, Bentzen SM, Deveau MA, Forrest LJ, et al. (2012) Spatially resolved regression analysis of pre-treatment FDG, FLT and Cu-ATSM PET from post-treatment FDG PET: an exploratory study. Radiother Oncol 105(1): 41-48.
- Douglas JG, Stelzer KJ, Mankoff DA (2006) [F-18]-fluorodeoxyglucose positron emission tomography for targeting radiation dose escalation for patients with glioblastoma multiforme: clinical outcomes and patterns of failure. Int J Radiat Oncol Biol Phys 64(1): 886-891.
- Piroth, Coenen HH, Kaiser HJ, Langen KJ, Eble MJ, et al. (2012) Integrated boost IMRT with FET-PET-adapted local dose escalation in glioblastomas:results of a prospective phase II study. Strahlenther Onkol 188(4): 334-339.
- Berwouts D, Olteanu LA, Duprez F, Vercauteren T, De Gersem W, et al.(2013) Three-phase adaptive dose-paintingby-numbers for head-and- neck cancer: initial results of the phase I clinical trial. Radiother Oncol 107(3): 310-316.
- Duprez F, De Neve W, De Gersem W, Coghe M, Madani I (2011) Adaptive dosepainting by numbers for head and-neck cancer. Int J Radiat Oncol Biol Phys 80(4): 1045-1055.
- Madani I, Duthoy W, Derie C, De Gersem W, Boterberg T, et al. (2007) Positron emission tomography-guided, focal-dose escalation using intensity-modulated radiotherapy for head and neck cancer. Int J Radiat Oncol Biol Phys 68(1): 126-135.
- Pinkawa M, Piroth, Jens Klotz, Victoria Djukic, Holy R, et al. (2012) Dose-escalation using intensity-modulated radiotherapy for prostate cancer: evaluation of quality of life with and without 18F-choline PET- CT detected simultaneous integrated boost. Radiat Oncol 7: 14.
- Van Elmpt W, De Ruysscher D, van der Salm A, Lakeman A, van der Stoep J, et al. (2012) The PET-boost randomizedphase II dose-escalation trial in non-small cell lung cancer. Radiother Oncol 104(1): 67-71.
- Jeraj R, Bradshaw T, Simoncic U (2015) Molecular Imaging to Plan Radiotherapy and Evaluate Its Efficacy. J Nucl Med 56(11): 1752-1765.
- Jeraj R (2016) Molecular imaging for radiotherapy optimization. Radiother Oncol 119(S1): S234.
- Petit SF, Van Elmpt WJ, Oberije CJ, Vegt E, Dingemans AM, et al. (2011) [8F] fluorodeoxyglucose uptake patternsin lung before radiotherapy identify areas more susceptible to radiationinduced lung toxicity in nonsmall-cell lung cancer patients. Int J Radiat Oncol Biol Phys 81(3): 698705.
- Eichinger M, Tetzlaff R, Puderbach M, Woodhouse N, Kauczor HU (2007) Proton magnetic resonance imaging for assessment of lung function and respiratory dynamics. Eur J Radiol 64(3): 329-334.
- Simon BA (2000) Non-invasive imaging of regional lung function using x-ray computed tomography. J Clin Monit Comput 16(5-6): 433-442.
- Ireland RH, Bragg CM, McJury M, Neil Woodhouse, Stan Fichele, et al. (2007) Feasibility of image registration and intensity-modulated radiotherapy planning with hyperpolarized helium-3 magnetic resonance imaging for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 68: 273-281.
- Lei CJ, Xu l, Sun Y J (2012) 18F-fluoromisonidazole PET/CT: a potential tool for predicting primary endocrine therapy resistance in breast cancer. J Nucl Med 53: 182-190.
- Cheng J, Lei L, Xu J, Yifei Sun, Yongping Zhang, et al. (2013) 18F-Fluoromisonidazole PET/CT: A Potential Tool for Predicting Primary Endocrine Therapy Resistance in Breast Cancer. J Nucl Med 54(3): 333-340.
- Venema CM, Apollonio G, Hospers GA, Schröder CP, Dierckx RA, et al. (2016) Recommendations and Technical Aspects of 16α-[18F] Fluoro-17ß-Estradiol PET to Image the Estrogen Receptor In Vivo: The Groningen Experience. Clin Nucl Med 41(11): 844-851.
- Yoon HJ, Kang KW, Chun IK, Cho N, Im SA, et al. (2014) Correlation of breast cancer subtypes, based on estrogen receptor, progesterone receptor, and HER2, with functional imaging parameters from 68Ga-RGD PET/CT and “F-FDG PET/CT. Eur J Nucl Med Imaging 41(8): 1534-1543.
- Linden HM, Dehdashti F (2013) Novel methods and tracers for breast cancer imaging. Semin Nucl Med 43(4): 324-329.
- Van Krutchen M, Galudemans AW, de Vries EF, Regina GH, Beets Tan, et al. (2012) PET imaging of estrogen receptors as a diagnostic tool for breast cancer patients presenting with a clinical dilemma J Nucl Med 53(2): 182-190.
- Perkovic MN, Erjavec GN, Strac DS, Uzun S, Kozumplik O, et al. (2017) Theranostic Biomarkers for Schizophrenia. Int J Mol Sci 18(4): 733.
- Kamps R, Brandao RD, Bosch BJ, Paulussen AD, Xanthoulea S, et al. (2017) Next-Generation Sequencing in Oncology: Genetic Diagnosis, Risk Prediction and Cancer Classification. Int Jour of Molecular Sciences 18(2): 308.
- Yahata N, Kasai K, Kawato M (2017) Computational neuroscience approach to biomarkers and treatments for mental disorders. Psychiatry and clinical neurosciences 71(4): 215-237.
- Wallner PE, Anscher MS, Barker CA, Bassetti M, Bristow RG, et al. (2014) Current Status and Recommendations for the Future of Research, Teaching, and Testing in the Biological Sciences of Radiation Oncology: Report of the American Society for Radiation Oncology Cancer Biology/ Radiation Biology Task Force, Executive Summary. Int J Rad Oncol Bio Phys 88(1): 11-17.
- Grimm J, Scheinberg DA (2011) Will nanotechnology influence targeted cancer therapy? Semin Radiat Oncol 21(2): 80-87.
- Naczynski DJ, Sun CG, Pratx G (2014) Development of X-Ray Luminescent Infrared Probes for Tumor Imaging. Int J Rad Oncol Bio Phys 90 (1): S796
- Chetty IJ, Martel MK, Jaffray DA, Benedict SH, Hahn SM, et al. (2015) Technology for Innovation in Radiation Oncology. Int J Radiat Oncol Bio Phys 93(3): 485-492.
- Hassanzaden P, Alyabi F, Dinarvand R (2017) Application of modelling and nanotechnology-based approaches: The emergence of breakthroughs in theranostics of central nervous system disorders. Life Sci 182: 93-103.
- Haie Meder C, Siebert FA, Potter R (2011) Image guided, adaptive, accelerated, high dose brachytherapy as model for advanced small volume radiotherapy. Radiother Oncol 100(3): 333-343.
- Sinha N, Cifter G, Sayo E, Kumar R, Sridhar S, et al. (2015) Brachytherapy Application With In Situ Dose Painting Administered by Gold Nanoparticle Eluters. Int J Radiat Oncol Bio Phys 91(2): 385-392.
- Guo J, Rahma K, He Y, Li L, Holmes J, et al. (2017) Gold nanoparticles enlighten the future of cancer theranostics. Int J Nanomedicine 12: 6131-6152.
- Pradeep P, Kumar P, Choonara YE, Pillay V (2017) Targeted nanotechnologies for cancer intervention: a patent review (2010-2016). Expert Opin Ther Pat 27(9): 1005-1019.
- Kaanders JAHM, Hoeben BAW, Troost EGC, J Bussink, WJG Oyen, et al. (2013) Molecular imaging as biomarker in future clinical trials: The proof of the pudding is in the eating. Radiother Oncol 106(2): S163.
- Valentini V (2014) Conformal, tailored and adaptive radiation oncology in the molecular biology and functional imaging era. Radiother Oncol 111(S1): S181.
- Kong FM, Ten Haken RK, Schipper M, Frey KA, Hayman J, et al. (2017) Effect of Midtreatment PET/CT-Adapted Radiation Therapy With Concurrent Chemotherapy in Patients With Locally Advanced NonSmall-Cell Lung Cancer A Phase 2 Clinical Trial. JAMA Oncol 3(10): 13581365.
- Grimm J, Scheinberg DA (2011) Will nanotechnology influence targeted cancer therapy? Semin Radiat Oncol 21(2): 80-87.
- Scheinberg DA, Grimm J, Heller DA, Stater EP, Bradbury M, et al. (2017) Advances in the clinical translation of nanotechnology. Curr Opin Biotechnol 46: 66-73.
- Bekelman JE, Deye JA, Vikram B, Bentzen SM, Bruner D, et al. Redesigning radiotherapy quality assurance: opportunities to develop an efficient, evidence-based system to support clinical trials--report of the National Cancer Institute Work Group on Radiotherapy Quality Assurance. Int J Radiat Oncol Bio Phys 83(3): 782-790.
- Fitzgerald TJ, Bishop Jodoin M, Bosch WR, Curran WJ, Followill DS, et al.(2013) Future vision for the quality assurance of oncology clinical trials. Frontiers Oncol 3: 31.
- Bentzen SM (2005) Theragnostic imaging for radiation oncology: dose- painting by numbers. Lancet Oncol 6(2): 12-17.
© 2017 Carlos A Perez. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






